Do All Ionic Compounds Conduct Electricity in Water?
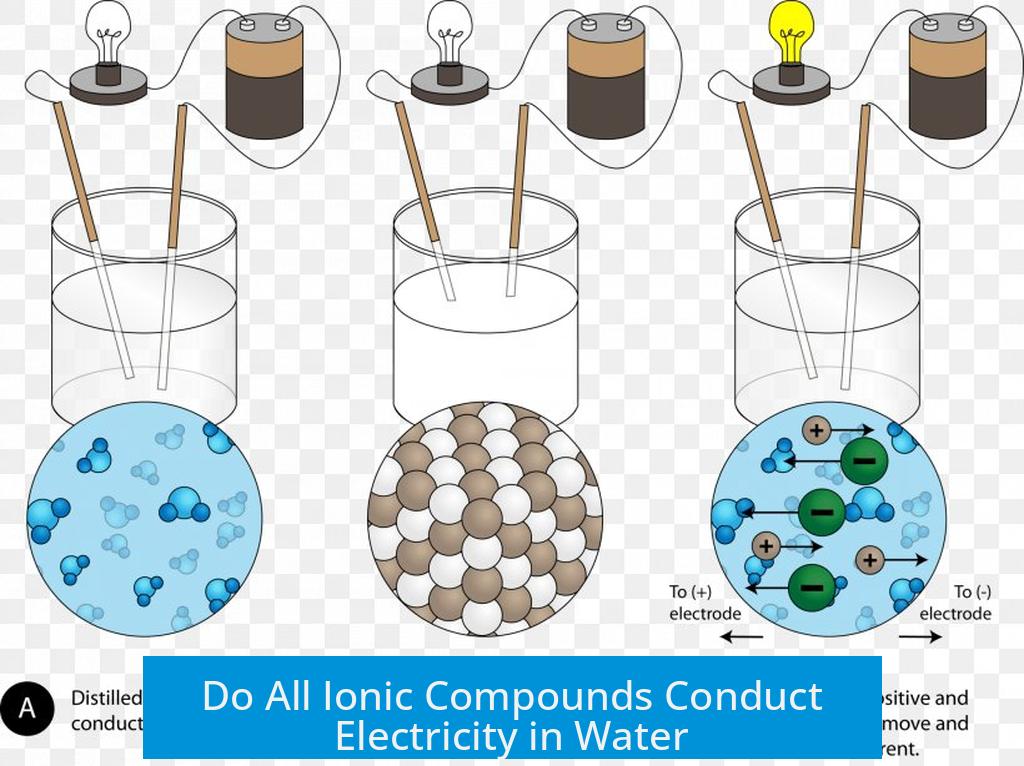
Not all ionic compounds conduct electricity when dissolved in water. The ability of an ionic compound to conduct electrical current in aqueous solution depends primarily on its solubility and degree of dissociation into ions.
Role of Solubility in Conductivity
Ionic compounds conduct electricity by releasing ions into water, forming electrolyte solutions. However, many ionic salts like barium sulfate (BaSO4), silver chloride (AgCl), and calcium fluoride (CaF2) are nearly insoluble in water. Because they do not dissolve well, these compounds fail to produce enough free ions to carry electrical charge, resulting in very poor or negligible conductivity.
Dissociation Extent and Conductivity
The ionic dissociation of a compound is not an all-or-nothing process. It exists on a spectrum where some salts dissociate almost completely, while others only partially ionize. This variation in dissociation affects the ionic concentration in solution and thus the solution’s conductivity. Conductivity can vary over many orders of magnitude between compounds.
- Highly soluble salts like sodium chloride (NaCl) dissociate fully, yielding strong conductivity.
- Weakly soluble salts provide very low ion concentrations and weak conductivity.
Influence of Water and Measurement Nuances

Water itself, even ultra-pure, contains trace ions that contribute to its low level of conductivity. Therefore, measuring electrical conductivity involves detecting differences above this baseline. Moreover, the interaction of water molecules with dissolved ions forms solvation complexes, further influencing conductivity.
Clarifying Misconceptions
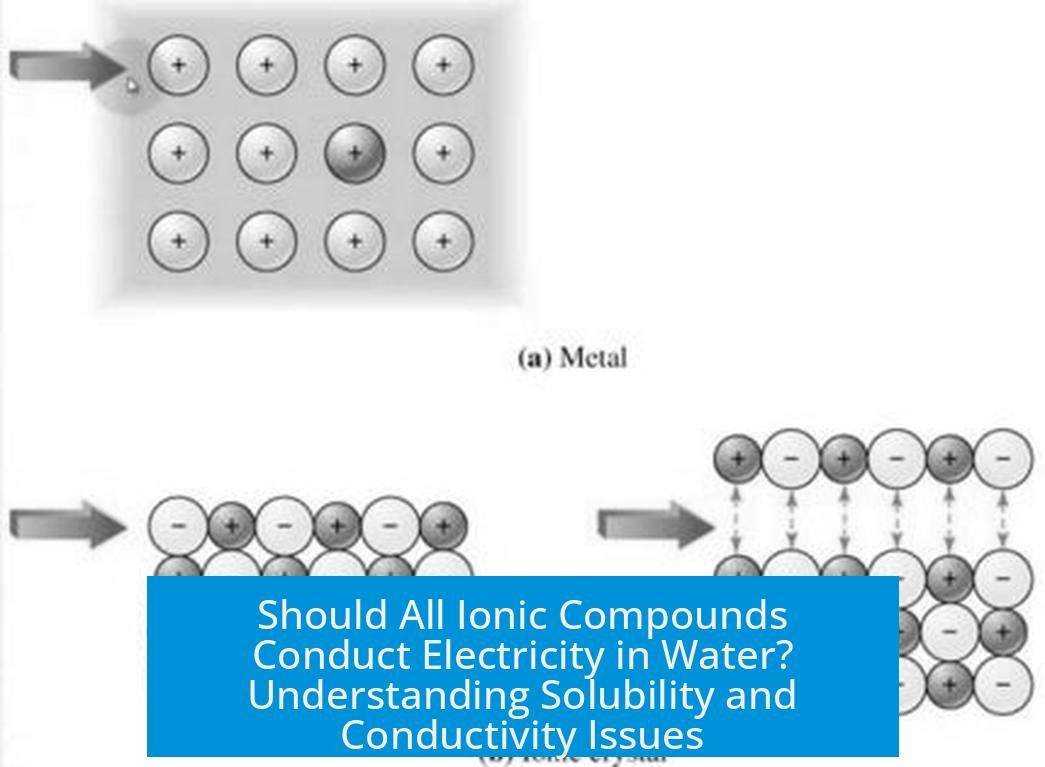
Conductivity in ionic solutions arises from the movement of charged ions, not from electron delocalization as seen in metals. This fundamental point distinguishes ionic conduction mechanisms in water from metallic conductivity.
Summary of Key Points
- Conductivity requires sufficient ionization; insoluble ionic compounds produce minimal ions and poor conductivity.
- Dissociation varies widely across ionic compounds, affecting conductivity levels on a continuum.
- Water always contains some ions; conductivity measurements must account for background levels.
- Conductivity mechanisms differ from electron flow in metals.
- Asking detailed questions about specific compounds or conditions yields more precise answers.




Leave a Comment