Niels Bohr’s Representation of Xenon
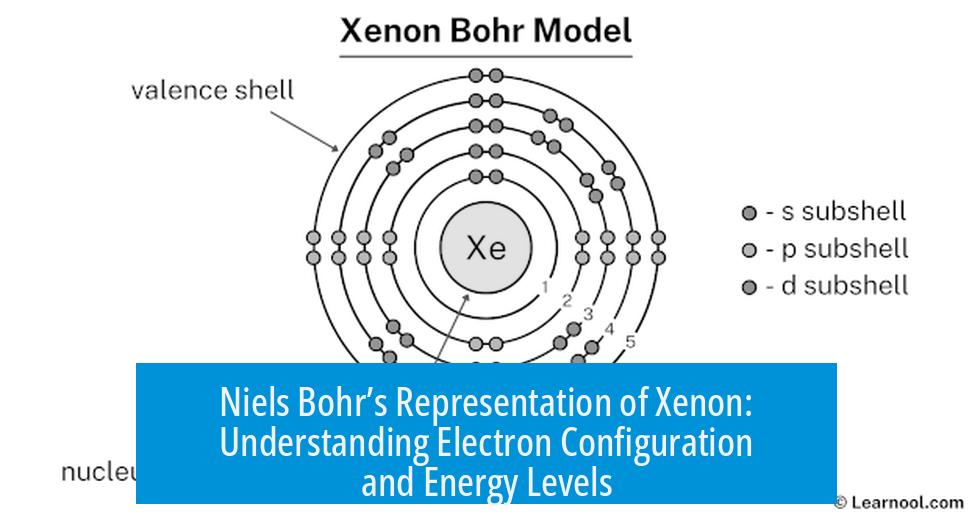
Niels Bohr’s representation of Xenon depicts electrons orbiting its nucleus in discrete circular energy levels, illustrating the noble gas’s electron configuration. The model emphasizes quantized energy states for Xenon’s 54 electrons but simplifies electron behavior compared to modern quantum theory.
Overview of Bohr’s Atomic Model
Niels Bohr introduced his atomic model in 1913. It portrays electrons traveling in fixed, circular orbits around the nucleus. Each orbit corresponds to a specific energy level. Electrons cannot exist between these levels but may jump from one orbit to another by absorbing or releasing energy.
Though influential, this view is an oversimplification. Modern science shows electrons behave more like probability clouds rather than particles moving in fixed paths. The exact position of an electron in an atom cannot be exactly determined, replaced by regions where an electron is likely to be found.
Applying Bohr’s Model to Xenon’s Electron Configuration
Xenon is a noble gas with atomic number 54, meaning it has 54 electrons. According to Bohr’s model, these electrons are arranged in shells around the nucleus. These shells correspond to energy levels numbered n = 1, 2, 3, and so forth.
| Energy Level (n) | Electron Capacity | Bohr Model Representation for Xenon |
|---|---|---|
| 1 | 2 | First orbit with 2 electrons |
| 2 | 8 | Second orbit with 8 electrons |
| 3 | 18 | Third orbit with 18 electrons |
| 4 | 18 (sometimes simplified to 8) | Fourth orbit with the remaining 18 electrons |
Bohr’s model places electrons in these clearly defined orbits. For Xenon, electrons fill the first three shells fully before filling the fourth. This depiction helps visualize Xenon’s stable, filled shell electronic structure typical of noble gases.
The Importance of Discrete Energy Levels
Bohr’s model introduced the concept that electrons occupy discrete energy levels. This means electrons cannot have arbitrary energies. They exist only at set distances from the nucleus where their energy is quantized.
This idea explains Xenon’s chemical stability. The outermost electrons in the highest occupied shell have a full complement, making electron transitions energetically unfavorable. The model captures this by showing filled orbits with no “empty” space for additional electrons.
Limitations of Bohr’s Model for Xenon
While Bohr’s model visualizes energy levels well, it lacks accuracy for complex atoms like Xenon. Xenon’s inner electrons exhibit electron-electron interactions and quantum mechanical effects that Bohr’s circular orbits cannot explain.
- Electrons in reality exist as wavefunctions spread over space.
- Electron orbitals are not simple circles but complex shapes due to quantum mechanics.
- Bohr’s model does not account for subshells (s, p, d, f) critical for Xenon’s chemistry.
Modern atomic theory uses quantum mechanics and Schrödinger’s equation to describe such atoms. These methods better explain electron clouds, probabilities, and multi-electron effects.
Educational Value of Bohr’s Representation
Despite its limits, Bohr’s model serves as a powerful educational tool. It simplifies the concept of electron shells and energy quantization for students and beginners. Xenon’s electron configuration, when presented with Bohr’s orbits, supports foundational learning of atomic structure.
Bohr’s model helps visualize:
- Why atoms emit or absorb light at discrete wavelengths.
- How electron transitions between energy levels relate to spectral lines.
- The concept of atomic stability tied to filled electron shells.
The model is often used in teaching VSEPR theory to explain molecular shapes, providing a stepping stone before introducing more advanced quantum chemistry.
Bohr’s Impact Relative to Earlier Atomic Theories
Bohr’s model represents a significant advancement in atomic theory beyond early ideas like Dalton’s indivisible atom concept. Dalton proposed atoms as solid spheres without internal structure.
Bohr introduced quantization, explaining spectral lines and the energetic behavior of electrons. For Xenon and other atoms, this basic framework paved the way for the vast developments in quantum mechanics and atomic spectroscopy.
Visualization and Artistic Appeal
Bohr’s representation of atoms, including Xenon, has also been visually iconic. Its clear concentric circles simplify complex quantum phenomena into engaging, understandable diagrams. Scientists and artists alike appreciate its aesthetic appeal and historical significance.
This visualization captures the imagination, encouraging interest in chemistry despite being scientifically outdated.
Complementing Bohr’s Model with Quantum Chemistry
Quantum chemistry supplements Bohr’s understanding, applying wave mechanics and molecular orbital theory to explain Xenon’s behavior more precisely. Hybrid approaches, combining Bohr’s model with Lewis bonding theory and molecular orbital concepts, cover up to 80% of basic chemistry effectively.
This pragmatic fusion supports chemists in predicting Xenon’s chemical interactions and inertness, using Bohr’s intuitive energy levels as a foundation while considering electron shielding and electronegativity at a more advanced level.
Summary of Key Points
- Bohr’s model represents Xenon’s electrons as orbiting in discrete energy levels.
- It emphasizes quantization of electron energy, explaining atomic stability and spectral lines.
- The model is simple and educational but oversimplifies electron behavior and orbits.
- Modern quantum mechanics replaces fixed orbits with electron probability clouds and complex orbital shapes.
- Bohr’s representation remains essential for teaching and initial understanding despite scientific limitations.
- Combining Bohr’s ideas with quantum chemistry provides a practical approach to understanding Xenon and chemical concepts.




Leave a Comment