Functional Group of Aldehydes
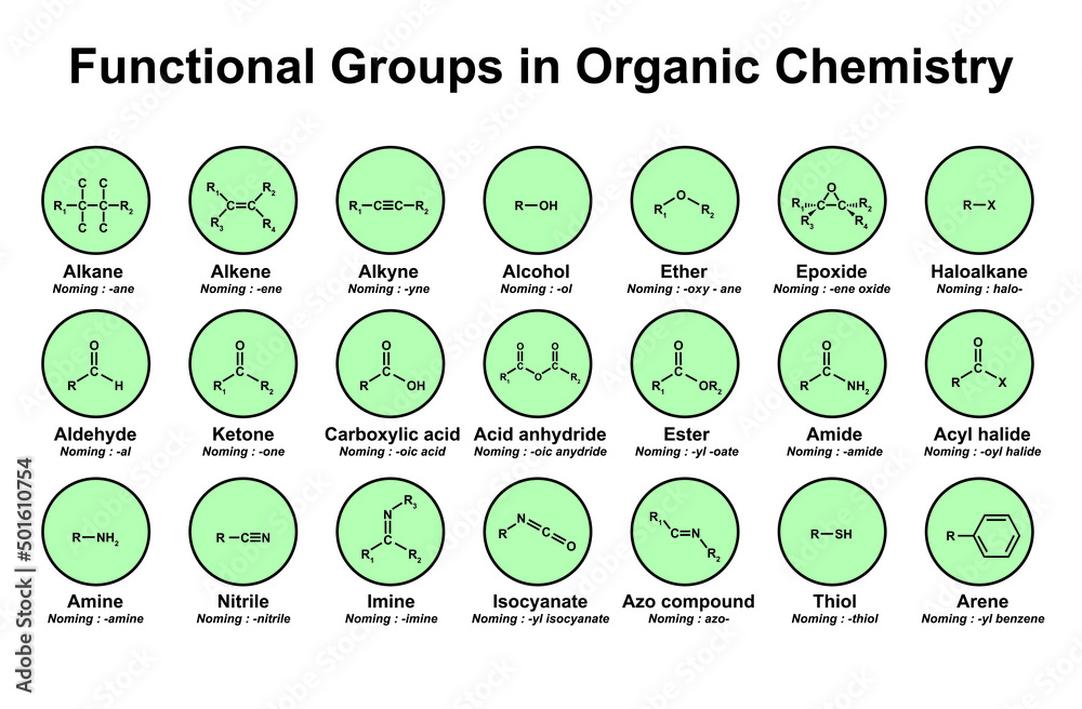
The functional group of aldehydes is the -CHO group, consisting of a carbonyl carbon atom bonded to a hydrogen and an R group. This distinguishes aldehydes from other carbonyl-containing compounds like ketones, which have two R groups attached to the carbonyl carbon.
1. Definition and Structure of the Aldehyde Functional Group
The aldehyde functional group includes a carbonyl group (C=O) directly bonded to at least one hydrogen atom. This is represented as -CHO in chemical formulas.
- Carbonyl group (C=O) is central but not unique to aldehydes.
- Aldehydes specifically have the carbonyl carbon bonded to hydrogen and one alkyl or aryl group (R–CHO).
- If the carbonyl carbon lacks a directly bonded hydrogen, the compound is not an aldehyde.
Ketones, in contrast, have the general structure R–CO–R, with two carbon groups attached to the carbonyl, hence not aldehydes.
2. Functional Group Versus Carbonyl Group
While the carbonyl (C=O) group is common to several functional groups, focusing solely on C=O leads to ambiguity.
- -CHO is the minimal and specific functional group for aldehydes.
- Carbonyl groups appear in ketones, aldehydes, amides, esters, and others, but their chemical behavior and identity differ.
- Functional groups denote specific reactive parts defining a compound’s chemical reactions.
Thus, the aldehyde group is more precisely described as -CHO rather than only C=O.
3. Functional Group Behavior and Transformation
The aldehyde group (-CHO) participates in characteristic reactions such as oxidation to carboxylic acids, reduction to primary alcohols, and nucleophilic addition.
Transformations occur consistently under defined reaction conditions, which helps classify -CHO as a functional group due to its predictable chemical behavior.
4. Nomenclature and Functional Group Terminology
There is some ambiguity in naming functional groups universally. For example, the -OH group can be called hydroxy or alcohol group depending on context.
For aldehydes, “aldehyde group” or “-CHO group” is widely accepted despite some nomenclature inconsistencies.
5. Historical and Contemporary Perspectives
Historically, some views, such as from about 20 years ago in Japan, considered aldehyde not as a functional group but simply as a chemical group.
Current perspectives generally recognize aldehyde as a functional group due to its distinct chemical properties and reactivity.
Key Takeaways
- Aldehyde functional group is specifically -CHO, not just C=O.
- Presence of hydrogen on carbonyl carbon defines aldehydes.
- Aldehydes react predictably, supporting their functional group classification.
- Nomenclature varies but -CHO remains the standard descriptor.
- Contemporary chemistry accepts aldehydes as functional groups.
The Lowdown on the Functional Group of Aldehydes: Why -CHO Rules the Roost
Ever wondered what makes aldehydes so special in the vast universe of organic chemistry? The secret lies deep inside their functional group. In simple terms, the functional group of aldehydes is -CHO, not just the carbonyl group C=O. Hang on! Before you say, “Wait, isn’t every aldehyde just a carbonyl?”, let’s unpack this nuance with clarity and a pinch of humor.
Think of the carbonyl group as the broad umbrella that shelters both aldehydes and ketones. Both flaunt a carbon double-bonded to oxygen. But aldehydes bring something unique to the garden party: the presence of a hydrogen atom directly bonded to that carbonyl carbon, forming the -CHO group. Ketones, in contrast, sport two carbon groups attached to the carbonyl carbon — no hydrogen included.
Carbonyl vs. Aldehyde Functional Group: Friends but Not Twins
The distinction here is crucial — and often misunderstood. While ketones are generally represented as -RC(=O)R, aldehydes live the simpler life: -RCHO. If the carbonyl carbon is missing its bonded hydrogen, you’re no longer in aldehyde territory.
This subtlety probably confuses many: “Isn’t C=O enough?” Nope. The presence of that hydrogen is what makes aldehydes unique. So next time you scan molecules in your textbook or lab, remember that the functional group of aldehydes isn’t just carbonyl but the specific -CHO assembly.
Why is -CHO the Functional Group in Aldehydes? Let’s Philosophy and Physics Enter the Chat
Now, you might ask: “Why -CHO? Why not just the carbonyl (C=O)?” Here’s where chemistry becomes a little philosophical — or at least deeply scientific. Functional groups aren’t only about atoms sticking together; they represent parts of molecules that behave consistently under certain reaction conditions.
The -CHO group transforms in predictable ways during chemical reactions. It reacts similarly under specific conditions — call them Y1, Y2, or Y3 in classic organic shorthand — which makes it functionally distinct from other carbonyl-containing groups like ketones or amides.
It’s like a signature dance move in the molecular ballroom. Functional groups “do” specific chemistry, and aldehydes have their unique choreography thanks to that bonded hydrogen. This almost poetic behavior traces back to electromagnetic forces operating at tiny length scales, between approximately 0.5 Ångström and 500 nanometers. That’s nano-sized style!
Cutting through the Nomenclature Fog: What’s in a Name?
Here’s where things get tricky, and your inner chemist might grunt in frustration. The International Union of Pure and Applied Chemistry (IUPAC), which many expect to be a beacon of clarity, doesn’t provide a crystal-clear standard for functional group naming. Sometimes, you’ll see the hydroxyl group called an “alcohol group,” and other times just the “hydroxy” group. Similar ambiguity pops up with the carboxyl/carboxylic acid groups.
For aldehydes, it’s usually safe to call -CHO the aldehyde functional group and “aldehyde” as the molecule type containing it — but keep your chemistry sense alert. Not everyone agrees, and tradition sometimes trumps scientific protocols.
History Lesson with a Twist: Japanese Chemists and Aldehydes
Here’s an amusing tidbit from the world of organic chemistry academia: About two decades ago, a Japanese organic chemistry professor revealed that aldehydes weren’t technically considered functional groups in Japan. They were just “aldehydes,” end of story.
Times change. Today, Japanese chemists commonly accept aldehydes as genuine functional groups, aligning with worldwide practices. This evolution reminds us that science is fluid and terms once disputed become standard over time. So if you feel a little lost in defining functional groups, you’re not alone!
Practical Tips for Identifying Aldehydes in Your Studies or Lab Work
- Look for the -CHO Group: Don’t be tricked by any lone C=O. Check if the carbonyl carbon has a hydrogen directly attached.
- Distinguish from Ketones: If both sides of the carbonyl carbon are bonded to carbons, it’s a ketone, not an aldehyde.
- Remember Functional Group Behavior: Aldehydes often oxidize to carboxylic acids, a key reaction that sets their chemistry apart.
- Use Nomenclature Wisely: When in doubt, specify “aldehyde functional group” to avoid confusion.
So, Why Care About This Nuance?
Understanding that the aldehyde functional group is -CHO and not just any carbonyl group equips you with sharper molecular insight. This distinction influences how aldehydes react, how they’re named in chemistry literature, and even how you ace your exams or lab reports!
Focusing on the -CHO group means your chemical intuition matures, helping you predict reaction outcomes better. For example, aldehydes typically participate in nucleophilic addition reactions more readily than ketones, thanks to that reactive hydrogen. In pharmaceuticals, this difference can mean the success or failure of creating a drug compound.
In Closing: Embrace the Quirkiness of Aldehydes
Here’s a quick recap to impress your peers or your next study group:
- Aldehydes have a special functional group called -CHO which includes a carbonyl with an attached hydrogen.
- Carbonyl groups alone aren’t sufficient to classify a molecule as an aldehyde.
- Functional groups are about consistent chemical behavior, not just atom arrangement.
- Naming conventions may confuse, but clarity comes from context and usage.
- Historical views vary but the modern stance embraces aldehydes as bona fide functional groups.
Next time you look at aldehydes, remember: they might seem simple, but their -CHO group holds the key to a universe of fascinating chemistry. Now, doesn’t that make you want to give a little molecular salute to the humble aldehyde?
What is the specific functional group that defines an aldehyde?
The functional group for aldehydes is -CHO. It includes a carbonyl carbon bonded directly to a hydrogen atom.
How does the aldehyde functional group differ from the generic carbonyl group?
While both aldehydes and ketones contain a carbonyl group (C=O), aldehydes must have the carbonyl carbon bonded to at least one hydrogen, making it -CHO.
Can we identify aldehydes simply by the presence of C=O in their structure?
No, the presence of C=O alone is not enough. Aldehydes specifically contain the -CHO group, meaning the carbonyl carbon has a hydrogen attached. Ketones and amides also have C=O but lack this hydrogen.
Is “aldehyde” universally accepted as a functional group name?
There is some ambiguity. In the past, some chemists, like those in Japan, did not consider aldehyde technically a functional group. Today, it’s generally accepted as one, though IUPAC naming guidelines can be unclear.
Why is the -CHO group considered functional in aldehydes?
The -CHO group undergoes characteristic reactions that define aldehydes. It transforms predictably under various chemical conditions, differentiating aldehydes from other carbonyl compounds.





Leave a Comment