Is the pKa of Methanol 0? Clarifying the Confusion
The pKa of methanol itself is not 0; it is approximately 15.5. The pKa value of 0 often cited refers to protonated methanol, a distinct species with much higher acidity.
Understanding pKa Values for Methanol and Its Protonated Form
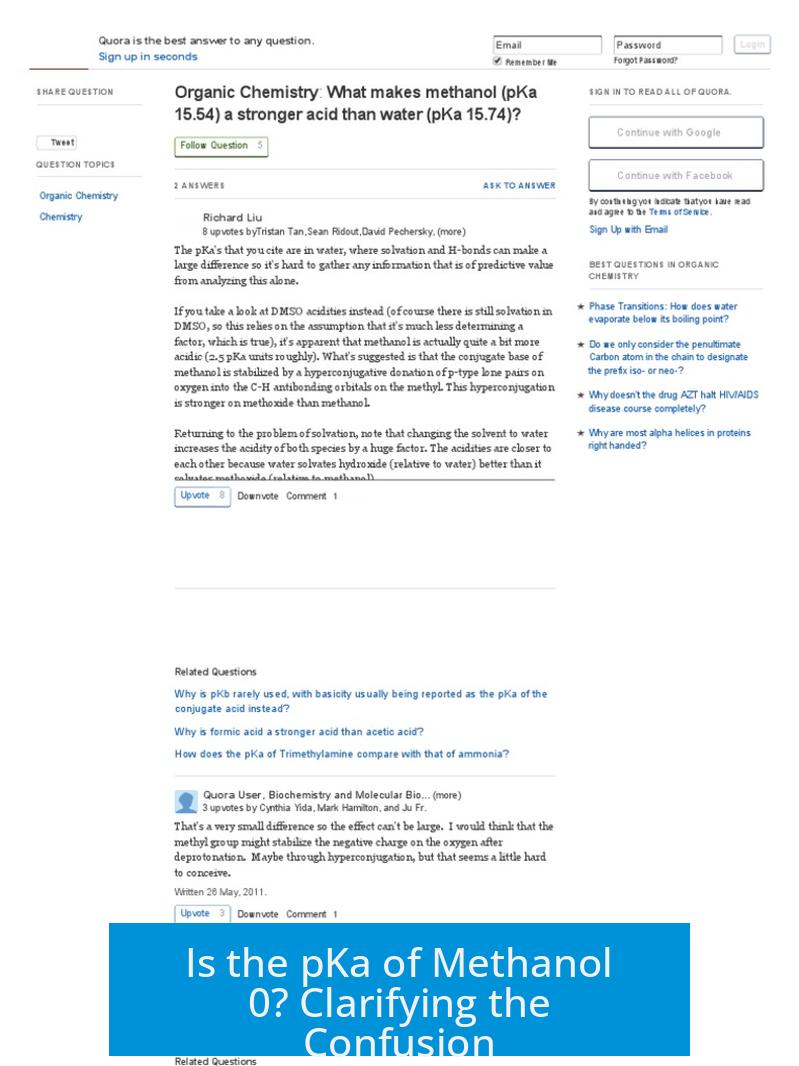
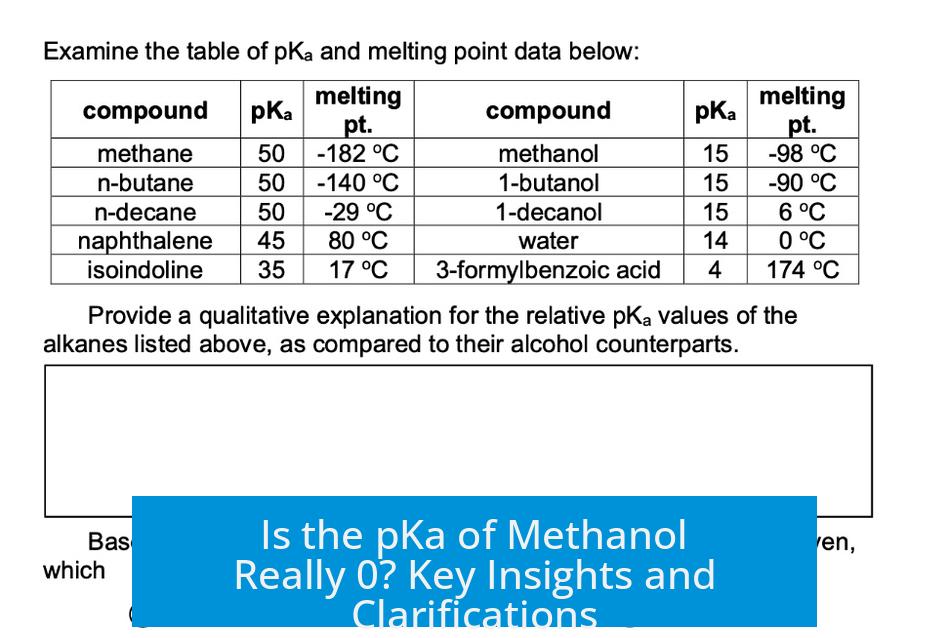
The pKa is a measure of acid strength related to the ease of donating a proton. Neutral methanol (CH3OH) has a pKa near 15.5. This means it is a very weak acid and does not easily lose a proton under normal conditions.
In contrast, protonated methanol (CH3OH2+), formed when methanol gains an extra proton, behaves as a strong acid. Its pKa is approximately -2.2, which is below zero and indicates high acidity.
Why Does the Answer Key Show pKa = 0?
The key source of confusion is that some answer keys list the pKa of the conjugate acid of a substance rather than that of the neutral molecule itself. For bases, chemists often refer to the pKa of their conjugate acids.
- Methanol’s conjugate acid is protonated methanol (CH3OH2+).
- This conjugate acid has a pKa near -2.2, close to zero in practical terms.
- The answer key’s pKa = 0 refers to this protonated form, not neutral methanol.
Summary Table of Relevant pKa Values
| Species | Chemical Formula | Approximate pKa | Description |
|---|---|---|---|
| Methanol | CH3OH | ~15.5 | Neutral alcohol, weak acid |
| Protonated methanol | CH3OH2+ | -2.2 | Conjugate acid of methanol, strong acid |
Key Points to Remember
- Methanol’s pKa is around 15.5, reflecting its weak acidity.
- Protonated methanol (conjugate acid) has a much lower pKa, near -2.2.
- Answer keys listing pKa = 0 typically refer to protonated methanol, not methanol itself.
- Confusion arises because pKa values can reference different species.




Leave a Comment