What is Solid-Phase Synthesis of Peptides?
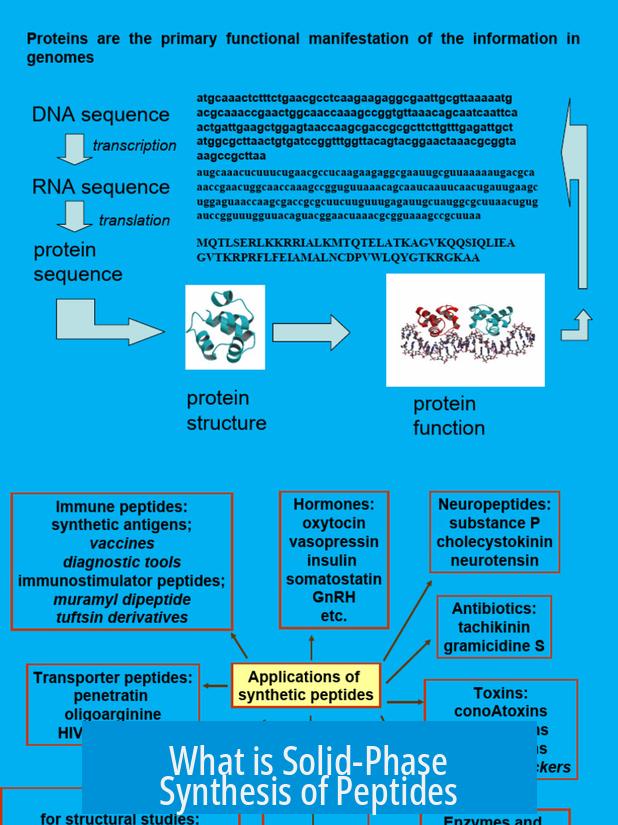
Solid-phase peptide synthesis (SPPS) is a method for creating peptides by sequentially attaching amino acids to a solid support resin, allowing efficient and rapid peptide assembly while simplifying purification steps.
Basic Concept and Process
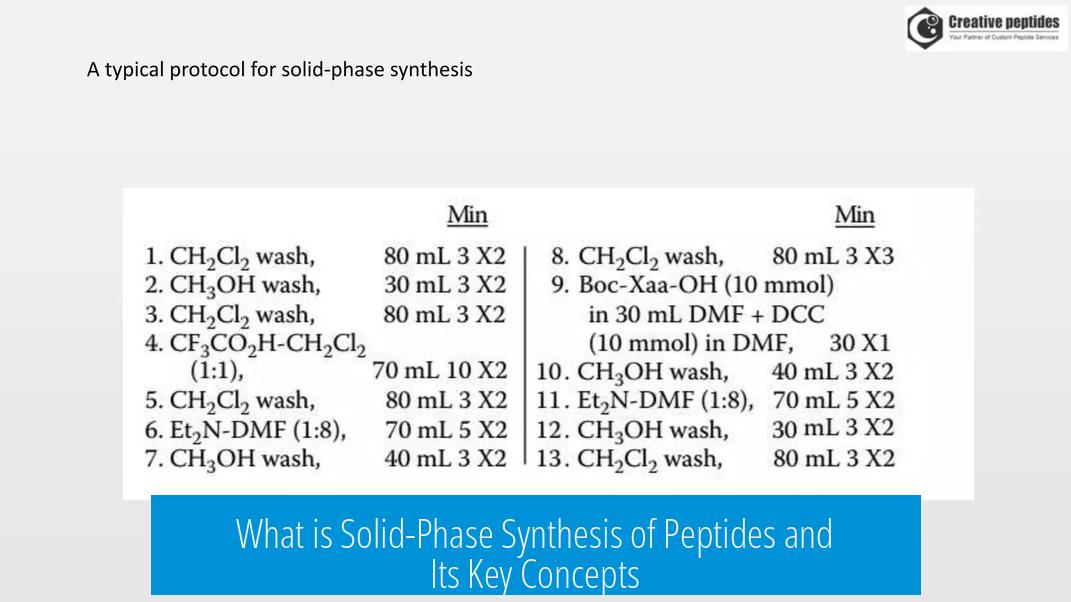
SPPS starts by anchoring the first amino acid to a solid resin at its C-terminus. This amino acid is protected at its amino group, commonly with groups like -Boc or -Fmoc, to prevent unwanted reactions during synthesis.
Peptide chain elongation happens by sequentially adding protected amino acids to the growing peptide bound on the resin. After each addition, the protecting group is removed using a strong base, which exposes the amino group for the next coupling. The system allows thorough washing to remove side products and unreacted reagents.
Cleavage and Final Peptide Recovery
Once the desired peptide sequence is complete, it is detached from the solid support. This is typically done by treating the resin-peptide complex with a strong acid like trifluoroacetic acid (TFA), which cleaves the bond connecting the peptide to the solid resin.
Advantages of Solid-Phase Peptide Synthesis
- Speed and Efficiency: SPPS enables rapid peptide assembly without isolating intermediates after each coupling reaction.
- Ease of Purification: Since the peptide remains attached to a solid resin, washing steps effectively remove excess reagents and byproducts, simplifying purification.
- Automation: The solid-phase approach facilitates automation, making it possible to produce peptides consistently and rapidly on an industrial scale.
- Improved Yield: Minimizing handling and isolations reduces losses and increases overall yield compared to solution-phase synthesis.
Historical Impact
SPPS revolutionized peptide chemistry. Its inventor, Robert Bruce Merrifield, received the Nobel Prize for this technique. The method transformed how peptides are synthesized worldwide, enabling advances in pharmaceuticals and biochemistry.
Summary of Key Points
- SPPS attaches and builds peptides on solid resin, simplifying synthesis.
- Protected amino acids are sequentially added starting from the C-terminus.
- Protecting groups are removed to allow chain elongation.
- Peptides are cleaved from resin using strong acids like TFA.
- The method is fast, efficient, and easily automated.
What is the main principle behind solid-phase peptide synthesis (SPPS)?
SPPS attaches the first amino acid to a solid resin. Amino acids are then added in sequence while linked to this solid support. The peptide grows step-by-step attached to the resin.
How are amino acids protected and added during SPPS?
Amino acids have protecting groups like Boc or Fmoc on their amino ends. These groups prevent unwanted reactions. After each addition, the protecting group is removed with a strong base before adding the next amino acid.
How is the finished peptide separated from the resin?
Once the peptide chain is complete, it is cleaved from the solid resin. This is done using a strong acid, often trifluoroacetic acid (TFA), to release the free peptide.
Why is SPPS considered more efficient than traditional methods?
SPPS allows fast synthesis without isolating the peptide after every step. Side products and excess reagents are washed away easily because the peptide stays attached to the resin, streamlining the process.
Can SPPS be automated and what benefit does that provide?
Yes, SPPS can be automated, enabling rapid and consistent peptide production. Automation reduces manual work and minimizes errors, making peptide synthesis more reliable and scalable.


Leave a Comment