How to Determine the Structure of a Compound with Formula C6H10
To figure out the structure of C6H10, combine the degree of unsaturation analysis with spectroscopic data interpretation, including IR, 13C NMR, and 1H NMR, then confirm possible candidates using spectral databases.
1. Calculate the Degree of Unsaturation
The formula C6H10 suggests unsaturation because a saturated alkane with six carbons is C6H14. The degree of unsaturation is:
- DU = (2C + 2 – H) / 2 = (2×6 + 2 – 10)/2 = (14 – 10)/2 = 2
This means there are two rings, two double bonds, or a combination such as a triple bond plus one double bond or ring.
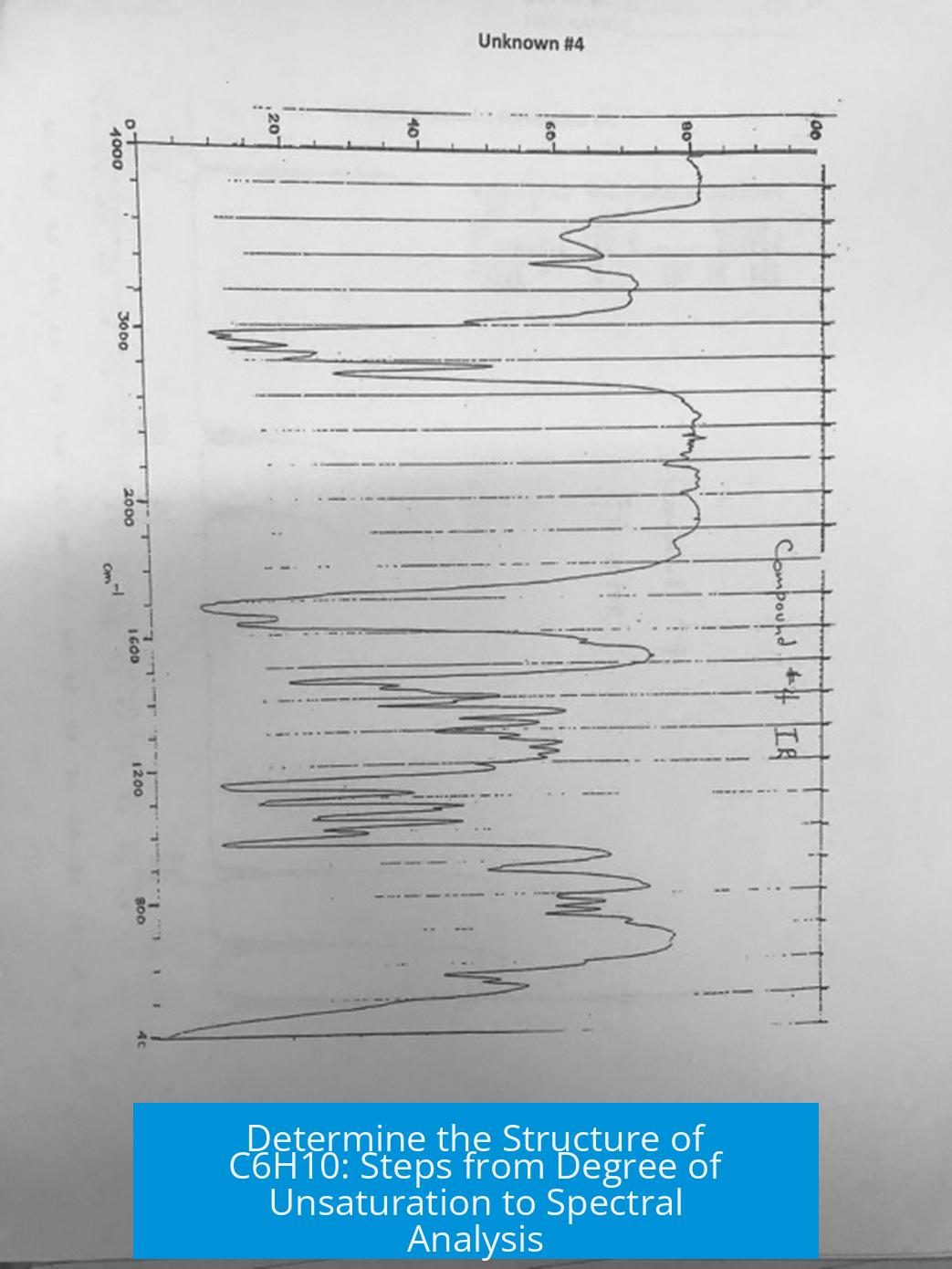
2. Identify Functional Groups with IR Spectroscopy
IR spectroscopy reveals functional groups. For C6H10, IR data can indicate an alkyne, especially if a terminal alkyne C-H stretch near 3300 cm-1 is observed. This narrows down structure possibilities to those with a triple bond.
3. Analyze Carbon Environments Using 13C NMR
13C NMR provides the number of unique carbon environments. Peaks around 85 and 68 ppm suggest sp carbons typical for alkynes. Counting these carbons aids in differentiating structural isomers.
4. Evaluate Hydrogen Environments with 1H NMR
1H NMR shows how many distinct hydrogens exist and their chemical environments. Splitting patterns reveal neighboring hydrogens and proximity to functional groups. Terminal alkyne hydrogen typically appears as a distinct singlet.
5. Confirm with Spectral Databases
Use the AIST spectral database (SDBS) to enter the formula C6H10 and the chemical shifts identified. Matching known 1H and 13C spectra helps pinpoint the correct structure among alkyne isomers.
Summary
- Determine the degree of unsaturation to assess rings or multiple bonds.
- Identify key functional groups with IR, focusing on alkyne signatures.
- Use 13C NMR to find distinct carbons and confirm alkynic carbons.
- Interpret 1H NMR to detail hydrogen environments and neighboring groups.
- Validate findings by comparison to spectra in trusted databases like SDBS.
How do I start figuring out the structure of a compound with formula C6H10?
Begin by calculating the degree of unsaturation. This tells you how many rings or multiple bonds the molecule may contain. It sets the stage for deeper analysis.
What role does IR spectroscopy play in determining the structure?
IR helps identify functional groups. For C6H10, detecting a terminal alkyne is common. This information guides you on possible bonding and shape.
How can 13C NMR help in structure determination?
13C NMR reveals how many unique carbons exist. It shows the types of carbon environments, like sp, sp2, or sp3 hybridized carbons. This narrows structural possibilities.
Why is 1H NMR important for figuring out the structure?
1H NMR tells you about different hydrogens and their surroundings. It helps locate hydrogens near functional groups and how they interact. This refines the proposed structure.
Can spectral databases assist in identifying the correct structure?
Yes. You can enter known chemical shifts and molecular formula into databases like the AIST spectral database. They suggest possible structures and let you compare spectra for confirmation.




Leave a Comment