The Lewis Dot Structure for HCN
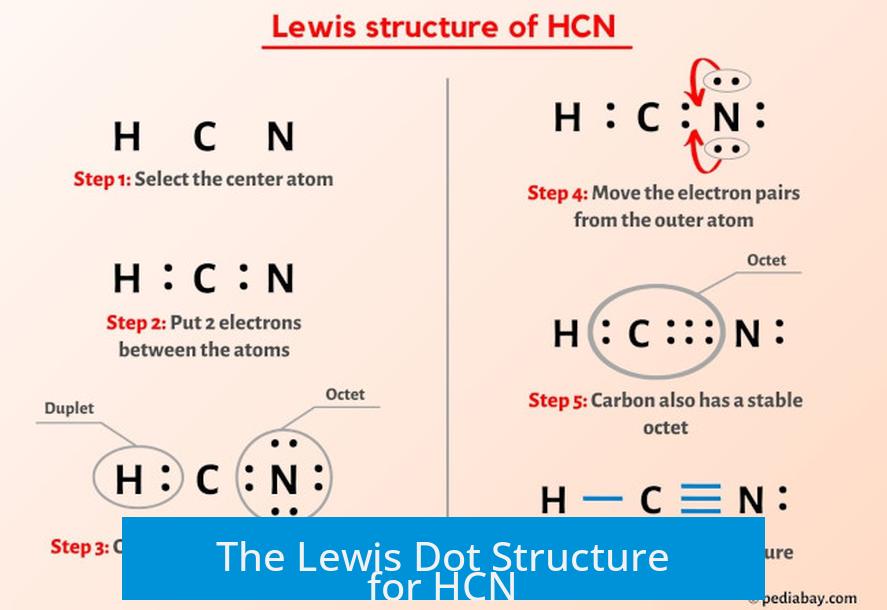
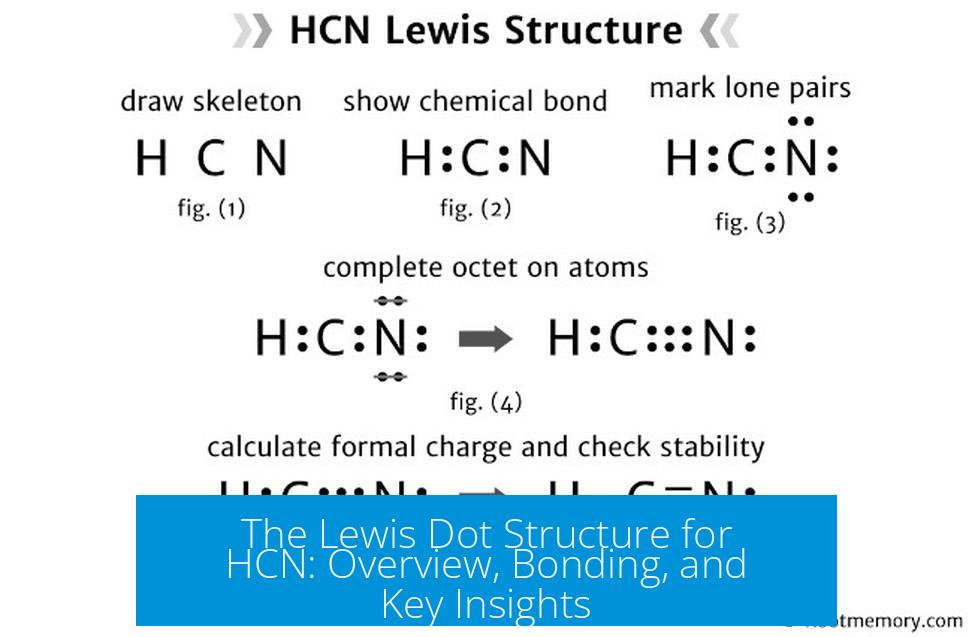
The Lewis dot structure for hydrogen cyanide (HCN) shows a linear molecule where the hydrogen (H) is bonded to carbon (C), which is triple bonded to nitrogen (N). This arrangement reflects the molecule’s valence electrons and bonding rules in a clear, concise format.
Structure Overview
- Hydrogen has one valence electron and forms a single bond with carbon.
- Carbon has four valence electrons and forms one single bond with hydrogen and a triple bond with nitrogen.
- Nitrogen has five valence electrons and shares three pairs with carbon in a triple bond, completing the octet.
These bonds illustrate electron sharing between atoms to fulfill the octet rule for carbon and nitrogen, and the duet rule for hydrogen.
Detailed Layout
| Atom | Valence Electrons | Bonds in HCN | Electrons for Octet |
|---|---|---|---|
| H (Hydrogen) | 1 | Single bond to C | 2 (duet satisfied) |
| C (Carbon) | 4 | Single bond to H, triple bond to N | 8 (octet satisfied) |
| N (Nitrogen) | 5 | Triple bond to C, one lone pair | 8 (octet satisfied) |
Electron Pairing and Bonding

Nitrogen carries one lone pair of electrons, which completes its octet beyond the triple bond with carbon. Carbon shares electrons with both hydrogen and nitrogen, making it a central atom in the molecule. The linear geometry follows from these connections and helps explain HCN’s molecular shape and polarity.
Context and Auxiliary Information
HCN’s Lewis structure is a key example in molecular chemistry, demonstrating multiple bond types in a small molecule. It is part of larger studies on molecular shapes and electron distributions. Such structures help predict reactivity and physical properties.
Suggestions for Further Details
- Include formal charge calculations for each atom to confirm stability.
- Discuss resonance structures, if any, and their relevance.
- Explain molecular orbital formation based on this structure.
- Relate structure to spectroscopic or bonding energy data.
Key Takeaways
- HCN features a single bond between H and C and a triple bond between C and N.
- Carbon and nitrogen each satisfy their octet; hydrogen completes its duet.
- The molecule is linear, reflecting the triple bond and bonding arrangement.
- Further exploration can include formal charges and resonance concepts.
What is the general Lewis dot structure for HCN?
HCN has a linear shape with a single bond between hydrogen and carbon. Carbon and nitrogen share a triple bond. Nitrogen also has a lone pair of electrons.
How do the bonds in HCN contribute to its stability?
The triple bond between carbon and nitrogen creates strong bonding. This bond and the single bond to hydrogen help keep the molecule stable and linear.
Why is nitrogen shown with a lone pair in HCN’s Lewis structure?
Nitrogen has five valence electrons. In HCN, three form bonds with carbon and two remain as a lone pair to satisfy nitrogen’s octet.
What role does the carbon atom play in HCN’s Lewis structure?
Carbon acts as a bridge between hydrogen and nitrogen. It forms one single bond with hydrogen and a triple bond with nitrogen, completing its octet.
Are there any common mistakes when drawing HCN’s Lewis structure?
One common error is not including the triple bond between carbon and nitrogen. Another is forgetting the lone pair on nitrogen, which is crucial for electron count.



Leave a Comment