How Chemists Actually Know the Shape of a Molecule
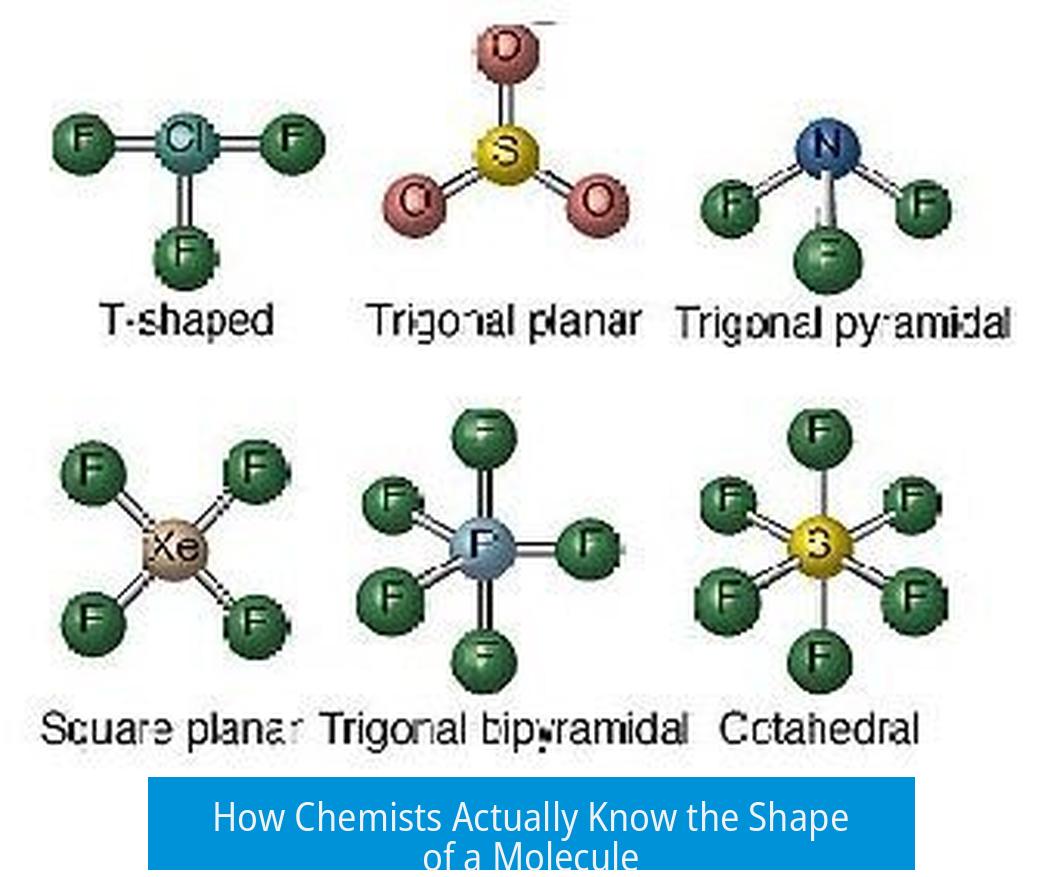
Chemists determine the shape of a molecule through a combination of experimental and theoretical methods. The key techniques include X-ray crystallography, NMR spectroscopy, rotational spectroscopy, computational chemistry, and advanced microscopy. Together these tools reveal both the molecular structure—how atoms are connected—and the three-dimensional shape or conformation the molecule adopts in space.
Distinguishing Structure from Shape
It is essential to clarify two related but distinct concepts: structure and shape. Structure refers to the connectivity of atoms—how atoms are bonded to one another. Shape (or conformation) describes the 3D arrangement of those atoms in space, including bond angles and lengths. For example, a molecule can have the same structure but exist in different conformations or shapes due to rotations around single bonds.
Primary Experimental Techniques
1. X-ray Crystallography
X-ray crystallography is the gold standard for directly visualizing molecular shape and structure. It involves crystallizing the molecule and analyzing the diffraction pattern produced when X-rays scatter through the crystal lattice. This method produces precise 3D atomic coordinates, showing both how atoms connect and their spatial arrangement.
One limitation is that crystallography captures just the solid-state shape, which may differ slightly from shapes in solution or gas phases. Also, molecules may crystallize in one polymorphic form, not revealing all conformations. Nonetheless, it provides high-resolution structural data.
Extensive databases like the Cambridge Crystallographic Data Centre (CCDC) provide access to crystal structures that illustrate diverse molecular shapes and packing patterns. This repository enriches understanding by comparing shapes across classes of molecules.
2. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy identifies chemical environments of atomic nuclei, principally hydrogen and carbon. The technique elucidates molecular structure by assigning peaks to specific atoms. More advanced 2D techniques (e.g., HSQC, HMBC) map connectivity across longer ranges.
While NMR excels at confirming structure, it only partially reveals shape. Nuclear Overhauser Effect Spectroscopy (NOESY) probes spatial proximity of atoms, offering clues about 3D arrangement. However, NOESY data can be ambiguous and incomplete, limiting conclusive shape determination.
3. Rotational (Microwave) Spectroscopy
Rotational spectroscopy measures energy transitions of molecules rotating in the gas phase. These transitions precisely depend on atomic positions relative to the molecule’s center of mass.
This technique can determine exact internuclear distances and angles for small molecules with remarkable accuracy. It complements other methods by refining positional data, especially where crystallography is impractical.
4. Mass Spectrometry
Mass spectrometry provides molecular weight and fragmentation patterns. Although it does not directly reveal shape, analysis of fragment ions informs on the structure. Isotope patterns assist in confirming molecular formulas.
Mass spectrometry cannot differentiate conformers; thus it is best combined with other techniques for comprehensive understanding.
Advanced and Complementary Methods
1. Computational Chemistry
Computational approaches use quantum mechanical calculations, such as Density Functional Theory (DFT), to predict the optimized molecular geometry. By minimizing the molecule’s energy along various bond lengths and angles, computational models identify the most stable shape under given conditions.
This method can simulate molecules in states difficult to isolate experimentally. It also aids interpretation of experimental data, bridging theory with observation.
2. Microscopy Techniques (AFM, STM, SEM, TEM)
Atomic force microscopy (AFM) and scanning tunneling microscopy (STM) can image large, flat molecules adsorbed on surfaces. These provide visual evidence of atomic arrangements and molecular packing.
However, these methods have limitations for small or flexible molecules. The requirement that molecules lie flat on a surface can distort their natural 3D conformation. Therefore, microscopy offers detailed surface views rather than true free-space shapes.
3. Neutron Diffraction
Neutron diffraction, akin to X-ray crystallography, uses neutrons scattered by atomic nuclei to determine structure. It excels at locating light atoms (such as hydrogen) within molecules, complementing X-ray analysis.
Historical and Theoretical Perspectives
Historically, early molecular structures such as Kekulé’s benzene model were speculative and lacked direct proof. Only later did experimental techniques validate these ideas. Modern methods depend heavily on combined spectroscopic data and theoretical models.
The determination of molecular shape is not solely direct observation but an integration of measurements, calculations, and chemical knowledge. This fusion allows chemists to infer 3D geometry reliably.
Summary of Techniques and Their Roles
| Technique | Information Provided | Strengths | Limitations |
|---|---|---|---|
| X-ray Crystallography | Definitive 3D structure and shape in solid state | High-resolution atomic positions | Requires crystallization; may not represent solution phase |
| NMR Spectroscopy | Chemical environments and connectivity; spatial data (via NOESY) | Non-destructive; works in solution | Partial shape info; NOESY can be ambiguous |
| Rotational Spectroscopy | Precise atomic distances in gas phase | Excellent accuracy for small molecules | Limited to small, gaseous species |
| Computational Chemistry | Predicted optimized molecular geometry | Explores states hard to isolate experimentally | Depends on computational model quality |
| Microscopy (AFM, STM) | Images of molecular surface and atomic arrangement | Visualizes larger planar molecules | Limited 3D shape accuracy; requires surface binding |
| Mass Spectrometry | Molecular weight and fragmentation | Highly sensitive; confirms composition | No direct shape info |
Key Takeaways
- Chemists distinguish molecular structure (connectivity) from shape (3D conformation).
- X-ray crystallography remains the most direct method for 3D shape determination.
- NMR and rotational spectroscopy provide complementary structural and spatial information.
- Computational chemistry predicts stable geometries by energy optimization calculations.
- Microscopy techniques offer visual insights but with limitations in true shape representation.
- Combining multiple methods yields the most reliable understanding of molecular shapes.
How do X-ray crystallography and NMR differ in revealing molecular shape?
X-ray crystallography produces a 3D map of a molecule showing exact atomic positions and shape. NMR reveals the chemical environment of atoms, helping with structure but offers limited direct shape information.
Why is rotational (microwave) spectroscopy important for small molecules?
It measures energy transitions between rotational states that depend on atomic positions. This method yields highly accurate atomic coordinates for small molecules.
Can computational chemistry alone determine the true shape of a molecule?
Computational methods predict stable molecular shapes by finding energy minima. They rely on theoretical models but need experimental data for confirmation.
Why don’t microscopy techniques like AFM or STM give full 3D molecular shapes?
They image molecules on surfaces, which can flatten or distort shapes. This limits their ability to show true 3D conformation, especially for small, flexible molecules.
How does mass spectrometry contribute to understanding a molecule’s structure and shape?
Mass spectrometry determines molecular weight and fragments molecules to infer bonding patterns. It helps identify the structure but does not directly reveal 3D shape.



Leave a Comment