How Can This Lewis Structure Be Correct When Placing a Positive Formal Charge on Oxygen?
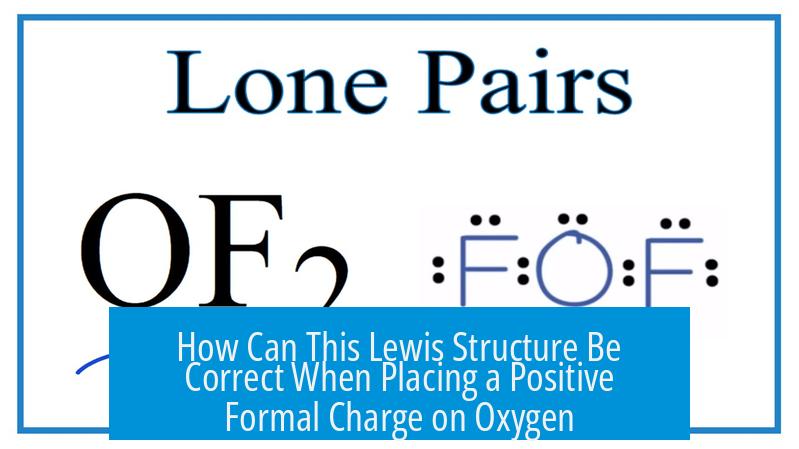
The Lewis structure that places a positive formal charge on the more electronegative oxygen in carbon monoxide (CO) is correct because it prioritizes filled octets over formal charge minimization, is supported by molecular orbital theory and experimental data, and reflects the actual electron distribution in the molecule.
1. Resonance Structures as Models, Not Absolute Truths
CO’s bonding is best described using multiple resonance structures. None fully portray the true electron distribution. Each resonance form is an approximation. Together, they offer an overall picture of the molecule’s electronic configuration.
One resonance structure shows oxygen with a positive formal charge, while carbon carries a negative one. Another form avoids formal charges but breaks the octet rule for carbon. Both are valid Lewis structures, but the one with full octets on both atoms, even with formal charges, is preferred.
2. Prioritizing Filled Octets Over Formal Charge Minimization
Chemistry textbooks emphasize two main criteria for preferred resonance structures:
- Atoms possessing filled octets are preferred.
- Resonance structures should minimize formal charges, placing negative charges on the more electronegative atoms when possible.
However, fulfilling the octet rule takes precedence over minimizing formal charges. In CO, the structure with oxygen having a positive formal charge ensures both carbon and oxygen complete their octets. A structure without formal charges leaves carbon deficient, which is less stable.
3. Limitations of Formal Charge and Electronegativity Rules
Formal charges are theoretical constructs designed to aid Lewis structure drawing. They do not strictly reflect actual charge distributions. The positive formal charge on oxygen does not mean it carries a full positive charge in reality. Instead, it signals a relative electron deficiency with respect to an idealized reference.
Electronegativity is a general guideline about electron affinity but does not strictly govern formal charge placement. For CO, although oxygen is more electronegative, electron donation towards carbon occurs, leading to oxygen displaying a slight positive partial charge. This contradicts simplistic electronegativity-based expectations.
4. Molecular Orbital Theory Offers a More Accurate Picture
Molecular orbital (MO) theory explains CO bonding better than Lewis structures alone. CO is isoelectronic with N2 and has a triple bond with a bond order of three. MO calculations show the bonding electrons are shifted slightly towards oxygen.
This electron distribution creates a small positive dipole on oxygen, consistent with the positive formal charge assigned to it in Lewis structures.
Furthermore, experimental measurements of CO’s dipole moment confirm oxygen as the positive end. This outcome validates the Lewis structure despite its counterintuitive formal charge placement.
5. Dative Bonding and Its Impact on Formal Charges
The positive formal charge on oxygen arises from a dative (coordinate) bond description. Here, oxygen donates a lone pair of electrons into an empty orbital on carbon, forming a third bond beyond the typical double bond.
This donation shifts electron density towards carbon, giving carbon a partial negative character and oxygen a positive one. The formal charge assignment reflects this electron shift.
This bonding explains CO’s ability to bind strongly to transition metals as a ligand, where carbon acts as the primary electron donor.
6. Understanding Lewis Structures as Simplified Models
Lewis structures are classical models predating quantum chemistry. They simplify complex electron interactions into discrete electron pairs, bonds, and charges.
They cannot fully capture partial charges or electron delocalization seen in real molecules.
In challenging cases like CO, compromises are common. Maintaining filled octets justifies accepting formal charges that appear to contradict electronegativity. The resultant structure is the best representation among imperfect choices.
Summary: Why the Lewis Structure with a Positive Formal Charge on Oxygen Is Correct
- Filled octets on both carbon and oxygen take priority over strict formal charge minimization.
- Formal charges are formal bookkeeping tools, not exact depictions of electron density or charges.
- Electronegativity trends do not always predict formal charge placement correctly in all molecules.
- Molecular orbital theory and dipole measurements show oxygen bears a slight positive dipole, matching the Lewis structure’s formal charges.
- Dative bonding from oxygen to carbon shifts electron density, causing oxygen’s positive formal charge.
- Lewis structures provide simplified models with limitations and should be treated as approximations, especially for molecules like CO.



Leave a Comment