How to Calculate an Rf Value
The Rf value is calculated by dividing the distance travelled by the substance by the distance travelled by the solvent front in chromatography. This ratio indicates how far a compound moves in a stationary phase relative to the solvent.
Definition of Rf Value
Retention Factor (Rf) is used in chromatography techniques like paper chromatography and thin-layer chromatography to quantify compound movement. It compares the distance a spot travels on a stationary medium with the solvent front’s travel distance.
Formula for Rf Value
- Rf = Distance traveled by substance / Distance traveled by solvent front
Here, the distance traveled by the substance is the length from the baseline (where the sample was originally placed) to the center of the spot. The solvent front distance is from the baseline to the point reached by the solvent’s leading edge.
Measurement Variables
- Distance by substance: Measured in centimeters or millimeters from the baseline to the middle of the spot.
- Distance by solvent front: Measured from the baseline to the solvent’s furthest point.
Multiple spots in a sample require measuring each spot’s distance individually.
Example Calculation

| Parameter | Value (cm) |
|---|---|
| Distance traveled by compound | 4.0 |
| Distance traveled by solvent front | 10.0 |
Using the formula, Rf = 4.0 / 10.0 = 0.4
Range and Interpretation of Rf Values
- Rf values always range between 0 and 1.
- An Rf near 1 means the compound travels nearly with the solvent, indicating high solubility.
- An Rf near 0 means little movement and low solubility in that solvent.
Factors Affecting Rf Values
- The choice of solvent strongly influences Rf values.
- Changing solvent polarity may increase or decrease compound mobility.
Practical Tips for Measuring Rf
- Measure distances vertically from the baseline using a ruler.
- Mark the solvent front immediately after chromatography to avoid errors from evaporation.
- Consider digital imaging tools like flatbed scanners or cameras for precise distance measurement.
Key Takeaways

- Calculate Rf as the ratio of compound distance to solvent front distance.
- Rf is dimensionless and ranges from 0 to 1.
- It aids in compound identification and solvent system optimization.
- Use accurate, vertical measurements from the baseline.
- Solvent choice directly impacts Rf values.
How to Calculate an Rf Value: A Simple Guide to Chromatography’s Favorite Number
Ever wondered how chemists separate mixtures and figure out who’s who in the world of molecules? One key player is the Retention Factor, or Rf value. This little ratio tells you how far a substance travels on a chromatography paper relative to the solvent front. Calculating it might sound complicated, but it’s really a straightforward process. Let’s dive into what it means and how to measure it accurately.
Chromatography, whether it’s paper chromatography or thin-layer chromatography (TLC), helps separate compounds based on how they interact with a stationary phase and a solvent. The Rf value is a handy metric to quantify those interactions.
What Exactly Is the Retention Factor (Rf)?
In simple terms, the Rf value measures how far a particular compound moves from the baseline compared to how far the solvent itself travels on the chromatography medium.
- Retention Factor (Rf) = Distance traveled by the substance ÷ Distance traveled by the solvent front
Think of it like a race where both the solvent and your compound start at the same line. The solvent tries to run the full length, and your compound’s Rf value tells you how far it’s come relative to that.
The Rf value is always a number between 0 and 1 because the compound cannot travel further than the solvent front. Zero means it barely budged; one means it ran with the solvent all the way.
The Formula in Action: How to Calculate Your Rf Value
It’s easy: just measure two distances—the spot’s movement and the solvent’s movement—from the baseline where you first applied your sample.
Rf = Distance traveled by substance / Distance traveled by solvent front
Imagine you observe the solvent front traveled 10 cm on your chromatography paper. You notice a blue dye spot traveled 4 cm up from the start point. Plugging in the numbers:
Rf = 4 cm / 10 cm = 0.4
This means the blue dye moved 40% of the solvent front distance. Simple, right?
Why Does Rf Matter? Unpacking Its Importance
Knowing the Rf allows scientists to:
- Identify substances—different compounds have distinct Rf values in the same solvent system.
- Compare experiments and ensure reproducibility.
- Optimize solvent systems to improve compound separation.
Would you trust a cake recipe without exact measurements? Similarly, a chemist can’t trust experiments without accurate Rf values.
How to Measure Distances Precisely (Because Accuracy Counts)
Here’s a golden nugget: mark the solvent front immediately after your experiment. Solvent tends to evaporate quickly, and guessing where it was leads you astray. Use a pencil to gently mark the solvent front as soon as you take the plate or paper out.
Next, grab a ruler and measure straight up—vertically—from the baseline to the center of your compound’s spot. Ignore sideways spreading. Why? Because chromatography is a vertical journey: it’s all about distance traveled upward.
Pro tip: For spots with fuzzy edges, measure to the center—not the edges—to keep your readings consistent and accurate.
Modern Twists: Measuring Rf Values with Scanners and Cameras
In more advanced labs, you might spot researchers using digital tools like flatbed scanners or digital cameras to capture and measure Rf values. Instead of eyeballing, these methods offer high precision and the ability to analyze multiple spots simultaneously. It’s the 21st-century touch to what used to be a ruler-and-pencil job.
Common Mistakes to Avoid When Calculating Rf Values
- Measuring after the solvent has dried up—leading to guesswork.
- Measuring along a diagonal instead of vertically—your Rf value won’t be accurate.
- Mixing units like centimeters and millimeters inconsistently.
- Comparing Rf values from different solvent systems without noting the solvent used.
Remember, Rf values only make sense when the solvent, the stationary phase, and the conditions remain consistent.
Interpreting Rf Values: What Does Your Number Say?
If your Rf value is close to 1, the compound is very soluble in the solvent and travels almost as far as the solvent front.
On the other hand, an Rf near 0 means the compound barely moved—likely it’s less soluble or interacts strongly with the stationary phase.
Want to find a compound that stays put? Look for very low Rf values. Need one that rides the solvent wave? High Rf is your friend.
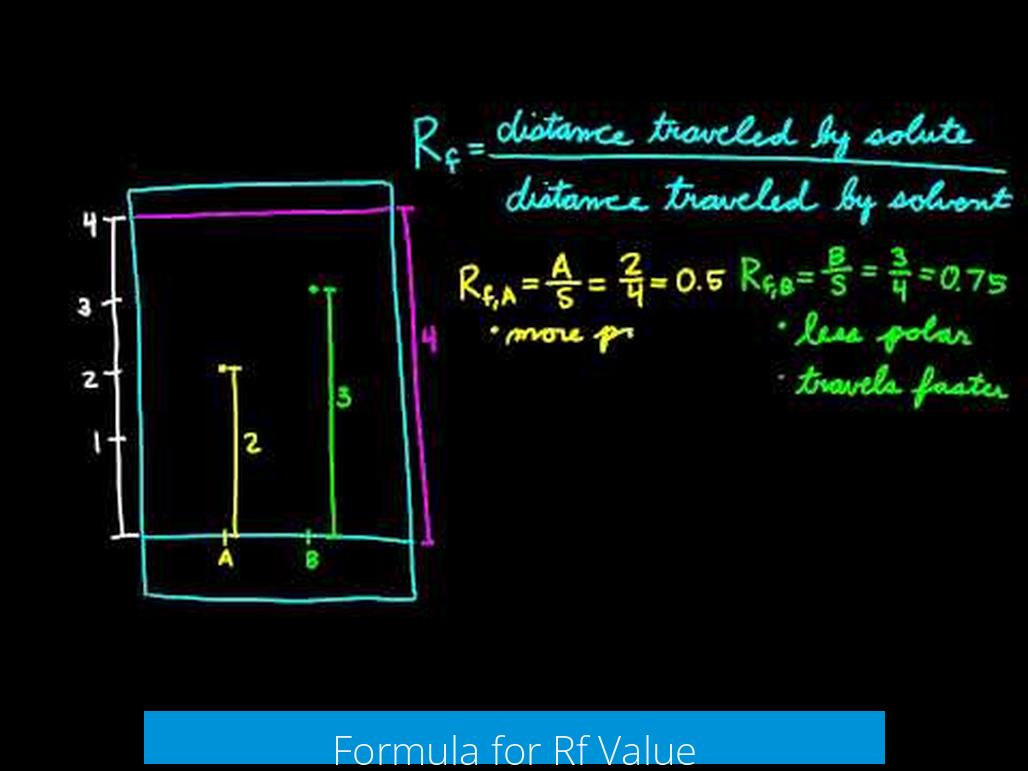
Real Example: Calculating Multiple Spots
Suppose you run an experiment, and your solvent front moves 12 cm. You spot three compounds that move 3 cm, 6 cm, and 9 cm respectively.
- Compound A: Rf = 3/12 = 0.25
- Compound B: Rf = 6/12 = 0.5
- Compound C: Rf = 9/12 = 0.75
These differences help you pinpoint each substance and compare with known Rf tables to identify them. Science feels like solving a puzzle, doesn’t it?
Final Thoughts: Measuring Rf Like a Pro
Calculating an Rf value is one of those skills that every budding chemist should master. It’s not just about numbers—it’s about reliable, reproducible data that tells a story about your compound’s chemistry.
Keep it simple: measure carefully, mark promptly, and calculate consistently. If you want to impress your lab partner, mention how digital imaging could improve your measurements someday!
Next time you see those colorful spots on a chromatography plate, you’ll know exactly how to transform them into meaningful numbers. And that’s the secret sauce for understanding and identifying mixtures.
What is the exact formula used to calculate an Rf value?
Rf is calculated with this formula: Rf = Distance traveled by the substance / Distance traveled by the solvent front.
Measure how far the compound moves and divide by how far the solvent moves in the same time.
How do you measure distances properly for Rf calculation?
Use a ruler to measure vertically from the baseline to the spot center for the compound’s distance.
Measure the solvent front distance immediately after the run before it evaporates.
Why can Rf values vary between experiments?
Rf values depend heavily on the solvent used and the stationary phase.
Changing the solvent or method often changes how far compounds travel, thus changing Rf.
What does an Rf value close to 1 or 0 indicate about a compound?
Close to 1 means the compound is very soluble in the solvent and moves far.
Close to 0 means the compound barely moves and is less soluble in the solvent.
Are Rf values unitless, and why?
Yes, Rf values are unitless since they represent a ratio of two distances.
This ratio compares how far the compound moves relative to the solvent front without units.




Leave a Comment