Isn’t 1 amu the Same as the Mass of a Proton or Neutron?
One atomic mass unit (amu), currently called the unified atomic mass unit (u), is not exactly the same as the mass of a proton or neutron. The term amu is outdated; the preferred term is “u.” Although a proton or neutron has a mass near 1 u, none of these particles have masses precisely equal to 1 u. The definition of 1 u is based on the mass of the carbon-12 atom, making it an average measure rather than an exact match to a single particle.
Understanding the Unified Atomic Mass Unit
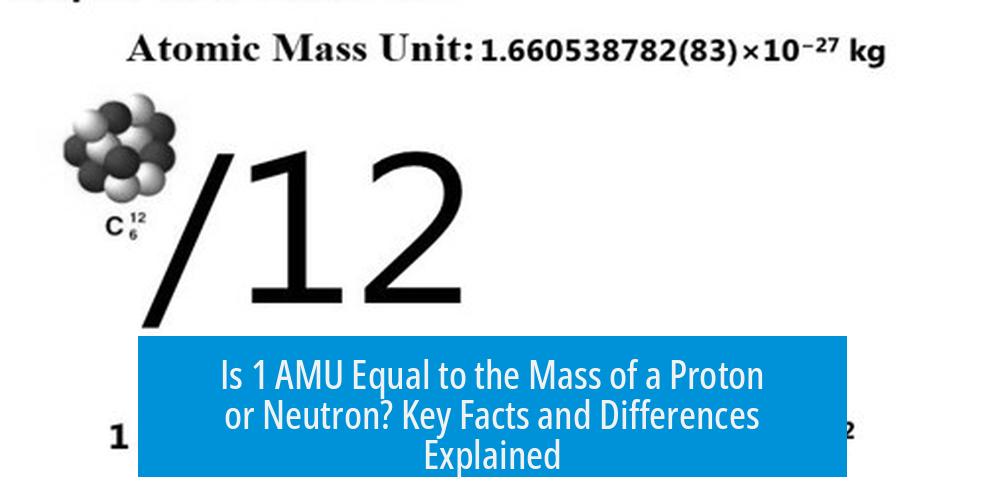
The unified atomic mass unit (u) standardizes atomic masses based on one twelfth the mass of a neutral carbon-12 (^12C) atom.
- 1 u = 1/12 the mass of a ^12C atom.
- This definition helps avoid measuring individual nucleons directly, which is more complicated.
- Using carbon-12 also balances the slightly different masses of protons and neutrons, and the minor contributions from electrons.
Because of this, 1 u acts as a convenient unit that closely aligns with proton and neutron masses but does not correspond exactly to either.
Mass Differences Between Protons and Neutrons
Protons and neutrons have different masses:
| Particle | Mass (u) |
|---|---|
| Proton | 1.0072765 |
| Neutron | 1.0086649 |
Thus, neither the proton nor neutron mass equals exactly 1 u. The neutron is slightly heavier than the proton.
The Mass Defect and Nuclear Binding Energy
Atomic nuclei masses are not simple sums of individual protons, neutrons, and electrons because of the mass defect.
- The total mass of nucleons in a nucleus exceeds the mass of the nucleus itself.
- The difference corresponds to the nuclear binding energy, the energy that holds the nucleus together.
- This energy converts to mass according to Einstein’s equation, E=mc2.
For example, lead-208 (^208Pb) contains 82 protons and 126 neutrons:
- Sum of proton masses: 82 × 1.0072765 u = 82.597853 u
- Sum of neutron masses: 126 × 1.0086649 u = 127.092383 u
- Sum of electron masses (82 electrons): 82 × 0.0005486799 u ≈ 0.045987 u
- Total naïve sum: ~209.736 u
The actual atomic mass of ^208Pb is 207.97665 u, about 1.76 u less than expected. This missing mass represents the nuclear binding energy released when nucleons combine.
Electron Mass and Binding Energy Contribution
Electrons have much smaller masses relative to nucleons, roughly 0.0005486 u each. Thus, their presence only slightly affects total atomic mass.
Electron binding energies—the energy locking electrons into atomic orbitals—are even smaller, typically negligible when compared to nuclear mass defects. For example, copper-63 (^63Cu) with a mass defect energy of 551.385 MeV has an electron binding energy near only 0.008% of that.
Why Is Carbon-12 the Basis for Atomic Mass Units?
Choosing the carbon-12 atom as the reference standard balances the uneven masses of protons, neutrons, and electrons. It also benefits from being easier to measure precisely.
This choice makes the atomic mass unit a practical, standardized concept useful in chemistry and physics. It also explains why 1 u does not exactly equal a proton or neutron mass, but is a carefully defined measure based on a whole atom’s mass.
Key Takeaways
- 1 amu (now u) equals 1/12 the mass of a carbon-12 atom, not the mass of a single proton or neutron.
- Protons and neutrons have slightly different masses: proton ≈1.0073 u; neutron ≈1.0087 u.
- Nuclear binding energy causes an atomic nucleus’s mass to be less than the sum of its parts; this is the mass defect.
- Electron masses and binding energies are much smaller and have negligible impact on total atomic mass.
- The carbon-12 standard provides a stable, precise base unit bridging particle mass variations.


Leave a Comment