The Kelvin, Celsius, and Fahrenheit temperature scales are fundamental to understanding temperature measurement, thermodynamics, and molecular motion. Kelvin represents an absolute scale tied directly to the energy state of particles, Celsius is widely used for everyday temperature, and Fahrenheit remains common in some regions. This article clarifies their definitions, relationships, and physical significance.
1. Fundamental Temperature Concepts: Absolute Zero and Molecular Motion

1.1. Absolute Zero: The Floor of Temperature
Absolute zero corresponds to 0 Kelvin (K). At this point, molecules theoretically have zero average kinetic energy. This means particles no longer exhibit motion associated with heat—the fundamental source of temperature.
In reality, quantum mechanics prevents particles from being perfectly still due to the Heisenberg uncertainty principle. This sets absolute zero as a theoretical limit rather than an achievable state. No particles can have less kinetic energy than zero, making 0 K the lowest possible temperature.
1.2. Molecular Interpretation of Temperature
Temperature measures the average kinetic energy of atoms or molecules in a substance. At temperatures above absolute zero, particles continually move, vibrate, and collide, transmitting heat.
The Boltzmann distribution describes how particle energies spread around an average. At 0 K, the distribution collapses so all particles have zero kinetic energy.
1.3. Special Cases: Negative Kelvin Temperatures
In specific, highly controlled quantum systems, formal definitions allow “negative” temperatures on the Kelvin scale. These occur due to an inversion of population in energy states but do not mean colder conditions. Instead, materials with negative Kelvin temperatures are considered hotter than any positive temperature.
1.4. Quantum Constraints Near Absolute Zero
Atoms don’t become perfectly motionless at 0 K due to quantum mechanics. Zero-point energy keeps particles in minimal residual motion. However, for most practical purposes, 0 K represents the cessation of thermal motion.
2. The Kelvin and Celsius Scales: Definitions and Conversions
2.1. Establishing the Kelvin Scale
The Kelvin scale arose from empirical observations related to gas behavior. Experiments measuring pressure (P) and temperature (T) at fixed volume (V) for gases revealed that plotting P versus T yields a linear relation.
Extrapolating this linear plot to zero pressure gave a temperature near -273.15 °C. This extrapolated zero pressure temperature defines 0 K, setting the starting point for the absolute temperature scale.
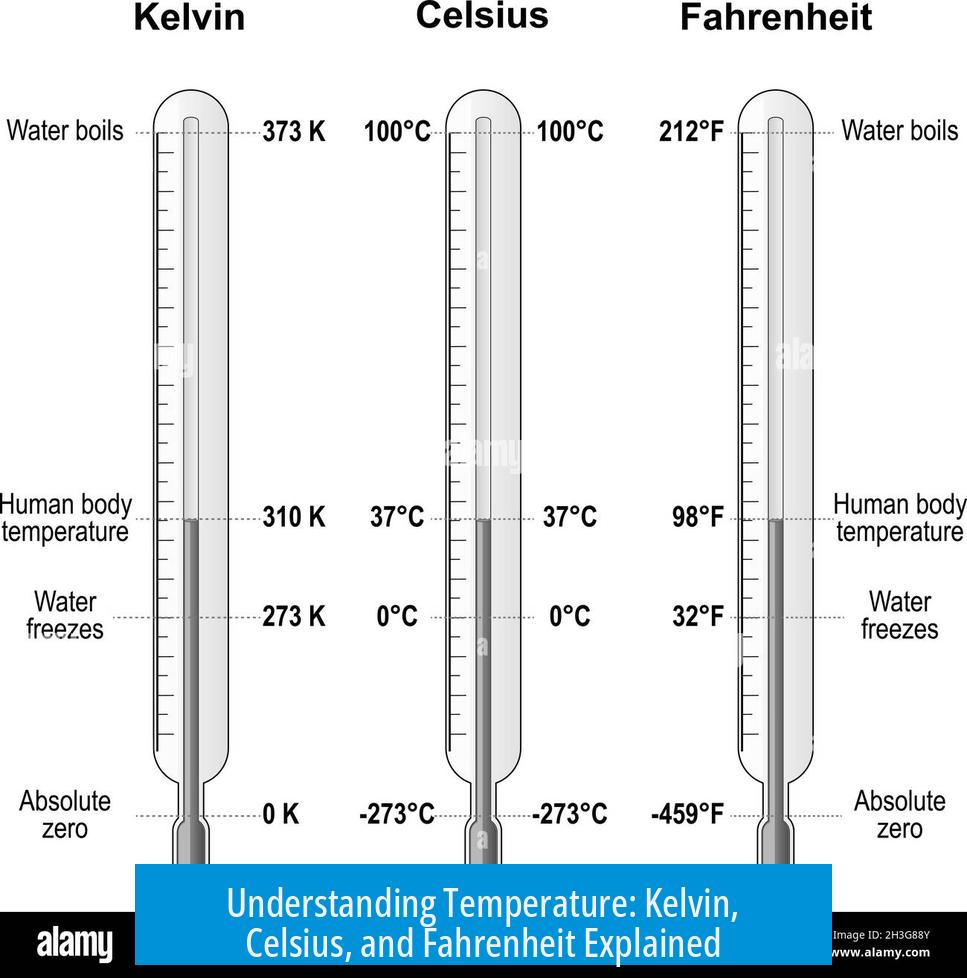
2.2. Relationship Between Celsius and Kelvin
| Scale | Definition | Relation |
|---|---|---|
| Celsius (°C) | Degrees relative to water’s freezing point (0 °C) and boiling point (100 °C) at standard pressure | Used commonly in weather, cooking, science |
| Kelvin (K) | Absolute temperature scale starting at absolute zero (–273.15 °C) | K = °C + 273.15 |
Conversion is straightforward: add 273.15 to Celsius to get Kelvin. This conversion prevents negative values in calculations like the ideal gas law PV = nRT, where temperature must be positive.
2.3. Kelvin Scale’s Role in Thermodynamics
Kelvin’s use in thermodynamics relates to its zero point representing absolute zero. It allows direct correlation between temperature and molecular energy. This empirical foundation simplifies problem-solving without delving into complex molecular models.
3. Fahrenheit Scale: Definition and Use
The Fahrenheit scale is primarily used in the United States. Defined such that water freezes at 32 °F and boils at 212 °F under standard conditions, it divides the interval between these points into 180 degrees.
Conversions between Celsius and Fahrenheit are given by: °F = (°C × 9/5) + 32
Although not derived from molecular properties, Fahrenheit remains relevant for everyday temperature reporting in some regions.
4. Heat and Temperature: Molecular Perspectives
Heat corresponds to the vibration and movement of atoms and molecules. When temperature rises, particles vibrate faster and collide more energetically. This molecular motion translates into thermal energy that we measure as heat.
A common analogy is particles acting like kids running in a playground. More movement means more heat; no movement corresponds to zero heat.
5. Summary of Key Relationships and Concepts
- Kelvin scale starts at absolute zero (0 K), the point where particles have zero average kinetic energy.
- Celsius scale is relative, based on water’s freezing (0 °C) and boiling (100 °C) points.
- Conversion from Celsius to Kelvin involves adding 273.15 (K = °C + 273.15).
- Fahrenheit scale sets water’s freezing at 32 °F and boiling at 212 °F; conversion to Celsius uses °F = (°C × 9/5) + 32.
- Absolute zero cannot be physically reached due to quantum uncertainty but serves as a theoretical ground point.
- Temperature measures average molecular kinetic energy; heat is the total energy from molecular motion.
6. Practical Implications in Science and Daily Life
Understanding these scales ensures proper temperature measurement and application in science, engineering, and daily activities. For instance:
- Scientific experiments require Kelvin measurements to maintain consistency and accuracy in thermodynamics.
- Weather forecasts use Celsius or Fahrenheit depending on the country, reflecting cultural preferences.
- Vacuum systems and cryogenics use Kelvin to approach conditions near absolute zero.
The fundamental insight is that Kelvin ties temperature to molecular energy in a physically meaningful way, unlike relative scales that serve convenience or tradition.
7. Conversion Table for Quick Reference
| Celsius (°C) | Kelvin (K) | Fahrenheit (°F) |
|---|---|---|
| -273.15 | 0 | -459.67 |
| 0 | 273.15 | 32 |
| 25 | 298.15 | 77 |
| 100 | 373.15 | 212 |
8. Clarifying Misconceptions
The idea that “molecules stop moving” exactly at absolute zero is an oversimplification. Quantum mechanics implies particles retain zero-point energy. But for practical and thermodynamic considerations, motion is minimal enough to represent a baseline for temperature measurement.
Negative Kelvin temperatures do not mean temperatures below absolute zero but rather unusual physical states under special conditions.
9. Conclusion
Kelvin, Celsius, and Fahrenheit scales serve different purposes. Kelvin aligns with physical laws and molecular behavior, Celsius offers convenience for everyday use, and Fahrenheit meets regional needs.
Temperature fundamentally connects to molecular kinetic energy, with absolute zero representing the theoretical minimum. Understanding their relationship ensures proper application in science and technology.
- Kelvin scale is absolute, based on molecular kinetic energy.
- Celsius is relative to water’s freezing and boiling points.
- Fahrenheit remains in use primarily in some countries, defined by common reference points.
- Conversion between scales is essential for scientific and practical accuracy.
- Absolute zero is unachievable but a critical theoretical limit in physics.
What is absolute zero in terms of molecular motion?
Absolute zero is the point where molecules have zero average kinetic energy. This means that molecular motion stops completely, although quantum effects prevent exact stillness.
Why is the Kelvin scale offset by 273.15 from Celsius?
This offset comes from gas law experiments. When pressure reaches zero at constant volume, the temperature is –273.15 °C, defining zero Kelvin as absolute zero.
Can temperature be below absolute zero?
Formally, some definitions allow negative Kelvin temperatures, but these do not mean colder than absolute zero. They are special cases and not actually colder states.
How is heat related to molecular motion?
Heat arises from the vibration and thermal motion of atoms and molecules. More motion means higher heat; no motion means no heat.
How does the Kelvin scale simplify gas law calculations?
Using Kelvin ensures absolute zero corresponds to zero pressure in the gas law PV = nRT. This makes calculations consistent and avoids negative temperatures.



Leave a Comment