Understanding Supersaturated Solutions: Clarifying Common Misconceptions

A supersaturated solution is one that contains more dissolved solute than its normal solubility limit at a given temperature and pressure. Merely adding excess solute, which precipitates out, does not make a solution supersaturated. The critical factor is how much solute remains dissolved, not how much solid is present.
Defining Supersaturation
Supersaturation refers to a state where the concentration of solute dissolved in a solvent exceeds the equilibrium solubility limit. This state is inherently unstable. In contrast, a solution with precipitated solute—solid particles settled out of solution—is not considered supersaturated.
It is important to distinguish between the amount of solute added to a solution and the amount actually dissolved. The solute that precipitates out is not part of the solution phase but forms a separate solid phase.
Why Adding Excess Solute Doesn’t Always Create Supersaturation
- Adding more solute than can dissolve creates a mixture of dissolved and undissolved solute.
- Only the dissolved solute contributes to the solution’s concentration relevant to saturation.
- Undissolved solute precipitates out and does not affect the solution’s saturation state beyond establishing equilibrium.
Therefore, simply having visible solid solute in a mixture does not mean the solution is supersaturated. The solution may be saturated if it holds the maximum amount of solute in solution, or undersaturated if less than the maximum amount is dissolved.
Quantitative Aspects: Saturation and Solubility
Scientists precisely define saturation using thermodynamics. The key measurement is the saturation ratio, which compares the product of dissolved ion concentrations to the solubility product constant (Ksp) of the solute.
| Condition | Saturation Ratio (Q/Ksp) | Solution State |
|---|---|---|
| Q/Ksp < 1 | Less than unity | Undersaturated (solute can dissolve more) |
| Q/Ksp = 1 | Equal to unity | Saturated (maximum solute dissolved) |
| Q/Ksp > 1 | Greater than unity | Supersaturated (more dissolved solute than equilibrium) |
Here, Q is the ion product calculated from dissolved solute concentrations; Ksp is a constant dependent on temperature and substance nature.
Role of Precipitate in Saturation State

Presence of precipitate indicates the solution is at or beyond saturation. When precipitate forms, it suggests the solution has reached equilibrium at saturation or has become supersaturated but is transitioning back to saturation.
Once precipitation occurs, the dissolved solute concentration decreases. Eventually, the solution reaches equilibrium at saturation, with excess solute precipitated out as solid.
Kinetics and Stability of Supersaturated Solutions
Supersaturated solutions are metastable. They can remain in this state temporarily due to energy barriers preventing immediate crystallization.
- The solute is in excess and seeks to precipitate but requires nucleation sites or disturbances.
- Triggering factors like adding seed crystals rapidly induce precipitation.
- This sensitivity explains why supersaturated solutions are rare and unstable in normal conditions.
This kinetic perspective explains why a solution can contain excess dissolved solute before precipitating out excess solid.
Reconciling the Teacher’s Statement with Standard Definitions
The statement that “a solution where too much solute has been added is a supersaturated solution even if the solute precipitates out” can cause confusion. According to chemical principles:
- Supersaturation strictly depends on dissolved solute exceeding solubility, not total solute added.
- If solute precipitates out, the solution is no longer supersaturated for that time period.
- Excess added solute that is undissolved forms a heterogeneous mixture, usually a saturated solution with solid present.
Hence, the teacher’s explanation lacks precision. Only when the dissolved concentration exceeds equilibrium limits, without immediate precipitation, does the solution qualify as supersaturated.
Summary: Key Points About Supersaturated Solutions
- Supersaturation requires more dissolved solute than normal solubility limits.
- Excess undissolved solute forming a precipitate does not define supersaturation.
- Solute must remain dissolved; precipitate is separate and indicates saturation.
- Supersaturation is thermodynamically unstable and prone to rapid precipitation.
- Quantitative saturation state compares dissolved solute concentration to solubility constants.
- Kinetic factors affect how long supersaturation persists before precipitation.
- Misunderstandings often arise by conflating added solute with dissolved solute.
Supersaturated Solutions: What Happens When Too Much Solute Joins the Party?
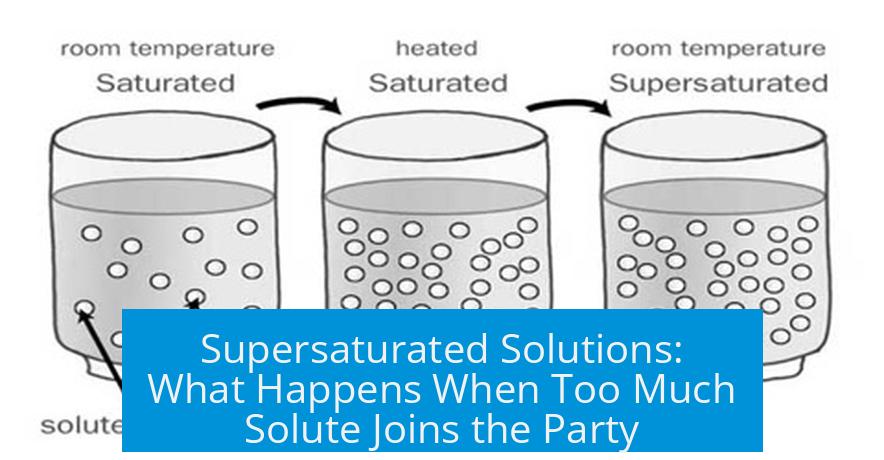
If you dump too much solute into a solution, is it automatically supersaturated? Not necessarily—and this is where chemistry throws a little curveball. Your teacher says a solution with too much solute—even if some of it falls out as solid—is supersaturated. Online definitions often say a supersaturated solution contains more dissolved solute than normal, with no undissolved particles hanging around. So, who’s right? Let’s dig into this sticky situation.
Chemistry isn’t just about mixing stuff randomly—it’s precise about what counts as part of the solution. The defining factor? How much solute is actually dissolved, not just added.
The Key: Dissolved Solute, Not Just Added Solute
Imagine you’re making a stubborn cup of sweet tea. You scoop more and more sugar into warm water. At first, the sugar dissolves readily; the tea sweetens. But eventually, sugar starts piling up at the bottom, refusing to dissolve any more. What just happened? You’ve reached the solution’s saturation point – the max amount of sugar water can hold at that temperature.
- Adding extra sugar beyond this point doesn’t make the solution supersaturated.
- The undissolved sugar is just sitting there as precipitate. It’s not part of the solution.
This is the critical misconception. Adding too much solute alone doesn’t create a supersaturated solution. Supersaturation requires more solute dissolved than the normal solubility limit.
What Exactly Is a Supersaturated Solution?
Supersaturation means the solution holds more dissolved solute than it “should” under normal conditions. This is a _delicate_ balance, like walking a tightrope.
Mathematically, scientists look at the ratio of dissolved solute concentration to its solubility constant (Ksp). When this ratio surpasses 1, you’ve got super saturation.
In simpler words: if the solution has more dissolved sodium and chloride ions than equilibrium allows, it’s supersaturated—and just waiting to drop some solute as solid!
Presence of Precipitate: Friend or Foe of Supersaturation?
Here’s a twist: a solution can have precipitate but still not be supersaturated. How? Well, if the undissolved solid is present, the solution portion (the liquid plus the dissolved solute) is generally at saturation, not supersaturation.
Think of it like this. Suppose you added too much salt to water, and some salt unconvincedly settles at the bottom. This leftover salt is not “activated” or dissolved. The solution can’t be considered supersaturated anymore since the dissolved content is at or below saturation.
Your teacher’s take—too much solute means supersaturation even if precipitate forms—fires up a debate but technically it misses a key detail. The solid precipitate is outside the solution phase and can’t count towards dissolved solute.
Why Do Supersaturated Solutions Exist at All?
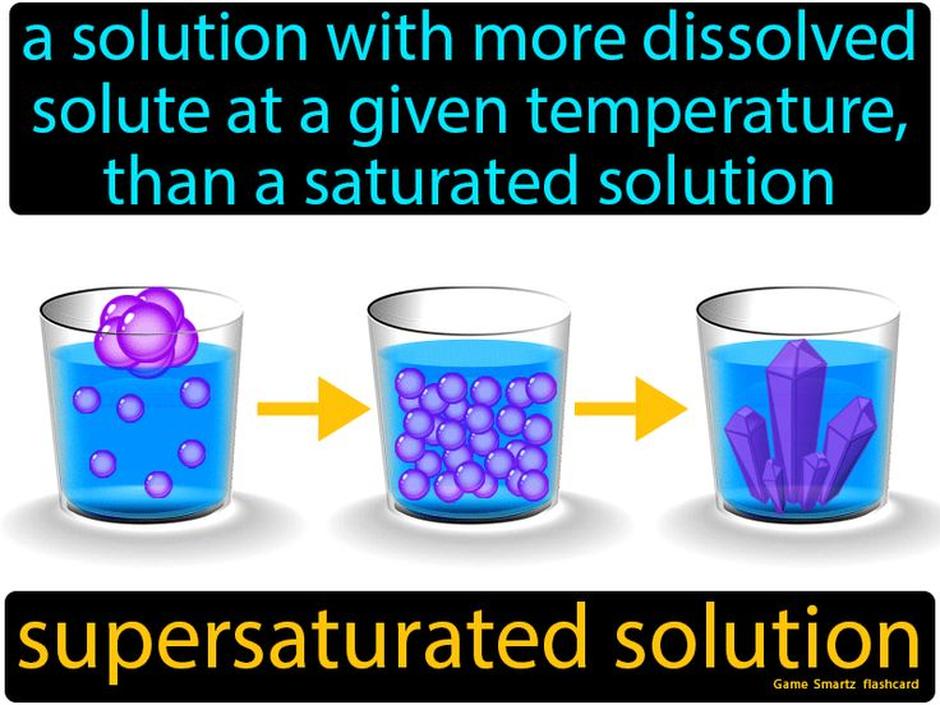
Natural solubility rules are strict, but kinetics like magic allow some solutions to hold extra solute temporarily. Supersaturated solutions are metastable. They can stay clear and stable for a fleeting moment, but they’re like a house of cards waiting for a gentle breeze.
For example, with sugar syrup: you heat sugar in water to dissolve more than the cold water could hold on its own. As the syrup cools carefully, the sugar remains dissolved beyond normal limits. But drop a sugar crystal inside, and boom—the sugary excess crashes out instantly.
This kinetic delay—the energy barrier solutes need to overcome to crystallize—lets supersaturation persist temporarily, but not forever.
Revisiting the Core Confusion
| Scenario | Supersaturated Solution? | Explanation |
|---|---|---|
| Extra solute added but undissolved (precipitate present) | No | Only dissolved solute counts. Precipitate means solution saturated or supersaturation lost. |
| Extra solute dissolved beyond normal saturation | Yes | Solution holds more solute dissolved than equilibrium allows, causing metastable supersaturation. |
The presence of precipitate signals the system is returning to equilibrium and **ceasing to be supersaturated**. You could say, precipitation is the solution’s way of self-correcting.
Practical Tip: How to Identify a Supersaturated Solution?
- Check clarity: supersaturated solutions often look clear, with no visible solids floating around.
- Test solute concentration: compare dissolved solute concentration against the solubility limit (Ksp) for that temperature.
- Introduce a seed crystal: if sudden precipitation occurs, a supersaturated state is likely.
If solids are visibly settled at the bottom right away, you’re likely dealing with a saturated solution plus a precipitate, not a supersaturated state.
When Teacher Meets Textbook: What to Do?
Teachers sometimes simplify concepts to build initial understanding. Your chemistry teacher’s comment might emphasize adding extra solute intuitively associates with supersaturation. But the accurate chemical definition is more rigorous, focusing strictly on dissolved amount.
Here’s a good approach:
- Respect your teacher’s insight—they often want you to think beyond simple cases.
- But also trust textbook definitions and scientific consensus which emphasize that precipitate means the solution is generally not supersaturated anymore.
- Ask your teacher for clarification or examples where precipitates coexist with supersaturation transiently—realistic in kinetic terms but fleeting.
Understanding these nuances sharpens critical thinking and experimental design, especially when you step from theory into lab work.
In Summary
A supersaturated solution is one where more solute is dissolved than normally possible under stable conditions. The presence of a precipitate means the extra solute is **no longer dissolved** and thus the solution isn’t supersaturated by strict chemical standards. Instead, you then have a saturated solution accompanied by solid particles.
So the next time you see a crystal settling at the bottom after adding too much sugar, remember, it isn’t supersaturation that you’re looking at—it’s just sweet-saturated water on the verge of crystallizing more.
And that little neat nuance? That’s chemistry keeping things interesting.





Leave a Comment