Why Hydrogen Doesn’t Have a Neutron

Hydrogen’s most common isotope, protium (1H), does not have a neutron because a single proton can form a stable nucleus on its own without requiring the presence of neutrons for stability. This is unique among elements, as nearly all other nuclei need neutrons to counterbalance proton-proton repulsion.
Stability of a Single Proton Nucleus
The nucleus of 1H consists of only one proton. This proton remains stable because, without additional protons, there is no internal electromagnetic repulsion requiring neutrons to provide nuclear glue. Neutrons serve primarily to mediate the strong nuclear force that holds multiple protons together in larger nuclei. Therefore, neutrons are unnecessary for the stability of hydrogen in its most abundant form.
Role of Protons and Neutrons in the Nucleus
- Protons carry a positive charge and repel each other through electromagnetic force.
- In nuclei with two or more protons, this repulsion must be balanced by the strong nuclear force, primarily mediated by neutrons.
- Neutrons act as a “glue” through the strong force to keep the nucleus stable despite proton-proton repulsion.
Since 1H contains only a single proton, there is no need for neutrons to hold the nucleus together, making it the only stable isotope without neutrons.
The Nuclear Force and Quantum Chromodynamics
The strong nuclear force between nucleons arises from quantum chromodynamics (QCD), where quarks inside protons and neutrons interact via color charge. The force is mediated by pions, particles resulting from quark-antiquark pairs, enabling nucleons to remain bound.
This interaction explains why adding neutrons to nuclei with multiple protons is essential but is not required when the nucleus is just one proton.
Hydrogen Isotopes and Neutron Presence
Hydrogen can have isotopes containing neutrons:
| Isotope | Composition | Neutron Count |
|---|---|---|
| Protium (1H) | 1 proton | 0 |
| Deuterium (2H) | 1 proton, 1 neutron | 1 |
| Tritium (3H) | 1 proton, 2 neutrons | 2 |
Adding neutrons creates these isotopes but does not affect the basic stability of protium.
Cosmological Context
From a cosmic perspective, 1H is the simplest and most abundant nucleus formed shortly after the Big Bang. It accounts for about 75% of the universe’s baryonic matter. Its abundance continues because no fusion processes immediately convert all hydrogen into heavier elements, preserving its simple proton-only nucleus.
Key Takeaways
- Protium (1H) consists of a single proton and no neutron.
- Neutrons stabilize nuclei by overcoming proton-proton repulsion in multi-proton atoms.
- Hydrogen’s single proton nucleus is stable without the need for neutron glue.
- Strong nuclear force, rooted in quantum chromodynamics, underpins nucleus stability.
- Hydrogen isotopes with neutrons exist but protium remains dominant.
Can Someone Explain to Me Why Hydrogen Doesn’t Have a Neutron?
Hydrogen’s most common form simply doesn’t have a neutron because it consists of just one proton, and that single proton is stable on its own—no neutron needed. Sounds straightforward, right? But why exactly? Let’s unravel this puzzler that even some science geeks trip over.
First, think of a hydrogen atom as a tiny solar system: one central proton—the “sun”—and one electron orbiting it. The building block of almost everything, hydrogen’s nucleus is the simplest of all, containing just a lone proton. Unlike other elements, it doesn’t need neutrons to make it work.
Why Most Nuclei Need Neutrons But Hydrogen Gets a Pass
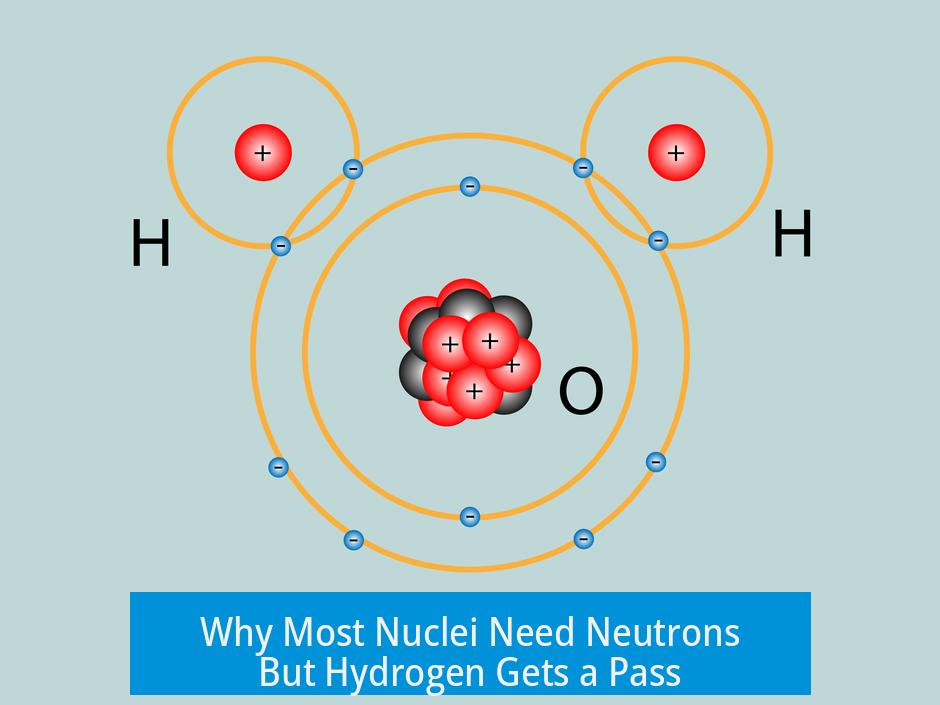
Neutrons act like the nuclear glue in pretty much every heavy nucleus. They help hold positively charged protons together.
- Protons, all positively charged, would repel each other hard enough to fly apart if they were alone.
- Neutrons come into the picture to mediate the strong nuclear force—a force stronger than electromagnetic repulsion, but only over tiny distances.
- Without neutrons, heavier nuclei couldn’t stay stable because the electrostatic repulsion between protons would tear them apart.
But hydrogen? It has just one proton in its nucleus—so no fellow protons to repel. This means the glue-neutron isn’t necessary. The nucleus is stable on its own.
The Role of Electromagnetic Force in the Hydrogen Neutron Mystery
It all boils down to electromagnetic repulsion between protons. Proton-positive beams repel like charges do. If there’s only one proton, this repulsion problem simply vanishes.
Imagine if you only had to keep one guest at a party from being annoying—you wouldn’t need a whole security team. That’s hydrogen! Other elements’ nuclei, with multiple protons, require neutrons to keep these positively charged guys in line.
You might ask: why are neutrons so good at this? It’s because they help mediate the nuclear force, a short-range force very strong but only effective inside the minuscule nucleus.
Quantum Chromodynamics—The Fancy Explanation With Quarks and Pions
Now, if we dive deeper into physics, protons and neutrons themselves aren’t elementary particles. They’re made up of quarks, held together by gluons. This falls under Quantum Chromodynamics (QCD), which explains the strong force.
Here’s where pions come in. They are like subatomic messengers—particles that pop in and out between nucleons (protons and neutrons) to keep the nuclear force balanced. Without a neutron, in hydrogen’s case, there’s no nuclear force dance needed because only one proton’s involved.
This complex dance of quarks and gluons ensures that nuclei heavier than hydrogen stick together despite the repelling force of positive charges. But hydrogen’s nucleus, with a solo proton, avoids this choreography entirely.
Hydrogen’s Other Isotopes: When Neutrons Do Join the Party
Now, don’t let hydrogen’s neutron-less status fool you. It can, and does, have neutrons in its heavier isotopes: deuterium (1H with one neutron) and tritium (1H with two neutrons).
These isotopes are less common but crucial for many scientific fields, including nuclear fusion research and radiometric dating. So, it’s not that hydrogen hates neutrons; rather, its simplest form—protium—just doesn’t require one to be stable.
Cosmic Origins: Why Hydrogen Is Neutron-Free in the First Place

The cosmic backstory is pretty amazing too. Early after the Big Bang, elementary particles like protons and neutrons emerged hot and fast. While neutrons started off floating freely, they quickly either decayed or joined protons to form more complex nuclei.
Since hydrogen’s nucleus is just a proton, it was the first and most abundant nucleus to form in our universe. In fact, hydrogen still accounts for about 75% of all normal matter. Not bad for a one-proton wonder!
Remember, fusion—the process that combines protons and neutrons—didn’t have enough time right after the Big Bang to make every proton gain a neutron. So, hydrogen remained neutron-free by nature and necessity.
What Does All This Mean For You?
Understanding why hydrogen doesn’t have a neutron is more than a fun fact. It helps you grasp:
- How atomic nuclei maintain balance
- Why hydrogen is unique among elements
- The fundamental forces at work in the subatomic world
- How cosmic history shaped the universe’s elemental makeup
So next time you hear someone ask, “Why doesn’t hydrogen have a neutron?” you can confidently explain that it’s all about stability, electromagnetic forces, and the unique simplicity of hydrogen’s single-proton nucleus. Plus, when it does add neutrons, it becomes fascinating isotopes like deuterium and tritium—demonstrating that hydrogen can put on a bit more complexity when the situation calls for it.
Quick Recap Table: Hydrogen and Its Nuclear Neutron Status
| Hydrogen Form | Number of Protons | Number of Neutrons | Stability |
|---|---|---|---|
| Protium (1H) | 1 | 0 | Stable |
| Deuterium (2H) | 1 | 1 | Stable |
| Tritium (3H) | 1 | 2 | Radioactive |
Ever wondered how something so simple as hydrogen forms the basis of everything? It all comes down to physics writ tiny: that single proton, alone but stable, kicking off the universe’s chemical story. And neutrons? They join the team only when complexity steps in.
So, the next time you’re sipping water (H2O) or marveling at the stars, remember that it all starts with a simple proton that doesn’t even need a neutron to hold its own.
Why does hydrogen-1 (¹H) nucleus not need a neutron to be stable?
Hydrogen-1 has only one proton. Without other protons nearby, there is no repulsion to overcome. Neutrons act as a glue between multiple protons, so hydrogen-1 stays stable alone.
How do neutrons help hold nuclei with multiple protons together?
Protons repel each other due to their positive charge. Neutrons provide the strong nuclear force that counters this repulsion, keeping the nucleus intact when there is more than one proton.
Can hydrogen have neutrons, and if so, what changes?
Yes, hydrogen can have neutrons, forming isotopes like deuterium (one neutron) and tritium (two neutrons). Adding neutrons changes the isotope but keeps the element hydrogen.
Why doesn’t a free neutron exist on its own like a proton?
Free neutrons are unstable and decay quickly. Protons in hydrogen are stable alone, so hydrogen-1 contains just a proton without needing a neutron for stability.
What role does quantum chromodynamics play in nuclear stability?
Quantum chromodynamics explains the strong force between quarks inside protons and neutrons. This force, mediated by particles like pions, helps bind nucleons, balancing charges and stabilizing nuclei.




Leave a Comment