What is the Point of Recrystallization and How Does it Work?

Recrystallization serves to purify a solid chemical by selectively dissolving it in a solvent and allowing the pure product to crystallize while impurities remain dissolved. This process removes minor contaminants that can interfere with chemical reactions or analyses.
Purpose of Recrystallization
Recrystallization is a crucial purification method in chemistry. It improves the chemical’s purity by separating the desired compound from impurities, which often hinder experimental accuracy or product quality. This technique is essential when minor impurities affect experimental outcomes.
Principle of Recrystallization
Dissolution of the Solid Product
The process begins by dissolving the solid containing the target compound in an appropriate solvent, usually at elevated temperature. Heating increases solubility, ensuring that all solid dissolves.
Selective Crystallization
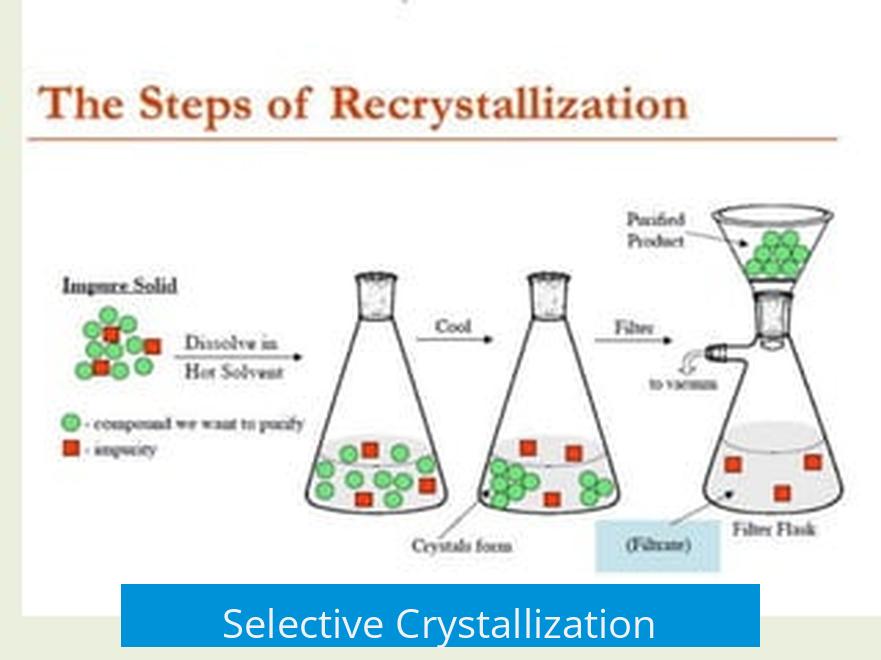
As the solution cools, the pure compound crystallizes out preferentially. Its molecules pack efficiently into a crystal lattice, while impurities remain dissolved since their molecular structures do not fit well into the crystal structure.
Purity Versus Yield
Recrystallization raises purity but often reduces yield. Some product is lost during the process due to dissolution and incomplete crystallization. Occasionally, impurities may crystallize first, reducing final purity; this is influenced by kinetic factors.
Practical Considerations
- Solvent Amount: Using too much solvent prevents crystals from forming as the compound stays dissolved. Minimal solvent volume optimizes crystallization. Heating the solvent after adding the product aids dissolution.
- Temperature Control: The hot saturated solution is filtered to remove insoluble impurities. Cooling, often with an ice bath, induces crystal formation.
- Solvent Selection: The solvent must dissolve the compound at high temperature but not at room temperature. The solvent’s polarity and coordination ability influence effectiveness. If the compound dissolves well at room temperature, alternative solvents or solvent mixtures should be tested.
- Product Recovery: Lost product can often be recovered by evaporating solvent to concentrate the solution before cooling again.
- Crystallization Aids: Applying vacuum can help remove solvent and promote crystallization.
Efficiency Compared to Other Methods
Recrystallization is simpler and less labor-intensive than techniques like column chromatography. It suits compounds that form well-defined crystals and have appropriate solubility properties.
Key Takeaways

- Recrystallization purifies solids by selective crystallization.
- It involves dissolving the product at high temperature and cooling to form pure crystals.
- Using the correct solvent amount and type is critical for success.
- Temperature control and careful filtration improve purity.
- Some product loss is common but can be minimized with proper technique.
- Recrystallization is a straightforward alternative to more complex purification methods.
What is the Point of Recrystallization and How Does it Work?
The point of recrystallization is to purify chemicals by selectively dissolving and then recrystallizing the desired compounds, leaving impurities behind in the solvent. This technique is crucial when tiny impurities can wreck your chemical reactions or experiments. Let’s explore how this process works, why it’s so valuable, and some practical tips to nail it in the lab.
Imagine you have a messy crystal of your target chemical. Recrystallization acts like a spa day for your compound—it dissolves everything into a hot solvent, then gently lets the best parts come back as pure, shiny crystals.
The Purpose: Why Bother With Recrystallization?
In chemistry, purity is king. When you have minor impurities sneaking around in your reagent, these little troublemakers can block reactions, produce unwanted side products, or send results into chaos. Recrystallization steps in as a purification hero. By removing those impurities, it clears the way for smoother, more reliable chemical work.
This purification step often matters more than volume or speed. After all, what’s the use of a large batch if it’s contaminated? Better to have less pure stuff than more mess.
How It Works: The Science Behind Recrystallization
Here’s the neat trick: you start by completely dissolving your impure solid in a solvent at a high temperature. Heating helps break the solid down so everything goes into solution. Then, as the solution cools, your target compound begins to form crystals again—but selectively.
This selectivity depends on molecular packing preferences. Your intended product molecules fit nicely together in a crystal lattice. In contrast, impurities don’t pack well and stay dissolved in the solvent. The slower you cool, the better the crystals can form neatly—excluding most impurities.
However, keep in mind the delicate balance between purity and yield. You’ll usually lose some of your product because not everything wants to crystallize perfectly. Sometimes, surprisingly, impurities crystallize faster! It’s a bit of a molecular race influenced by kinetics and molecular affinity. This can leave you paradoxically with less pure crystals if you aren’t careful.
Pro Tips: Mastering the Art of Recrystallization

Let’s get real about common pitfalls and how to avoid them.
- Solvent Amount: Too much solvent often spoils the party. If your crystals refuse to form, you probably drowned your product in excess solvent. Try using less solvent than you think you need and gently heat it as you add your product to dissolve it.
- Temperature Control: Most recipes call for heating the solution near its boiling point to fully dissolve your compound. Then comes the cooling part—often using an ice bath—to coax those crystals out of solution. Slow cooling produces better crystals.
- Solvent Quality: Ever heard of a solvent being “too good”? If your product dissolves in the solvent even at room temperature, consider trying a different solvent. The goal is to have your compound only dissolve well when hot, not when cold.
- Solvent Compatibility: Be cautious that your solvent doesn’t chemically react with your product or act too aggressively. Polar or coordinating solvents can sometimes create complex solubility behaviors that ruin your purification.
- Saving Lost Product: Did you “lose” your product in the solvent? Don’t panic! Evaporate the solvent carefully down to a smaller volume, then cool again to get your crystals back.
- Using Vacuum: When crystals stubbornly refuse to form, putting your solution under vacuum can help evaporate some solvent. This trick concentrates the solution and encourages crystal formation.
How Does Recrystallization Measure Up?
Compared to column chromatography, another common purification method, recrystallization scores points for simplicity. It doesn’t require fancy columns or endless solvents, just a beaker, the right solvent, and a little patience.
Is it perfect? No method ever is, but recrystallization often hits the sweet spot of cost, ease, and purity. Those who have tried both often praise its straightforwardness and reliability once mastered.
Wrapping It Up: The Beauty of Recrystallization
Recrystallization isn’t just a procedure—it’s an elegant dance between solubility, temperature, and molecular compatibility. Its goal is clear: to extract the best, purest crystals amid the impurities that cloud chemical experiments.
Want to improve your recrystallization game? Start with these questions:
- Is my solvent dissolving too much product at room temperature?
- Am I using the right amount of solvent, or drowning my sample?
- Have I controlled heating and cooling to allow slow crystal growth?
Apply these basics, and your lab work will thank you with cleaner compounds, better yields, and fewer headaches.
Indeed, the point of recrystallization is purity. And pure chemistry is good chemistry.
What is the main goal of recrystallization in chemical purification?
The goal is to purify chemicals by removing minor impurities. This step ensures the reagent does not interfere with the intended experimental results.
How does recrystallization separate pure product from impurities?
The solid product dissolves fully in a solvent when heated. Upon cooling, the pure product crystallizes out, leaving impurities dissolved in the solvent.
Why is controlling the amount of solvent important in recrystallization?
Too much solvent prevents crystals from forming because the product stays dissolved. Using less solvent and heating helps dissolve the product without hindering crystal formation.
What role does temperature play in recrystallization?
Typically, the solution is heated to dissolve the product fully. Then it is cooled, often with an ice bath, to allow pure crystals to form.
Can you recover product lost during recrystallization?
Yes. Evaporating some solvent reduces volume and encourages crystallization, allowing recovery of product lost in solution.




Leave a Comment