1H-NMR Analysis of Cyclopropylamine Hydrochloride Salt
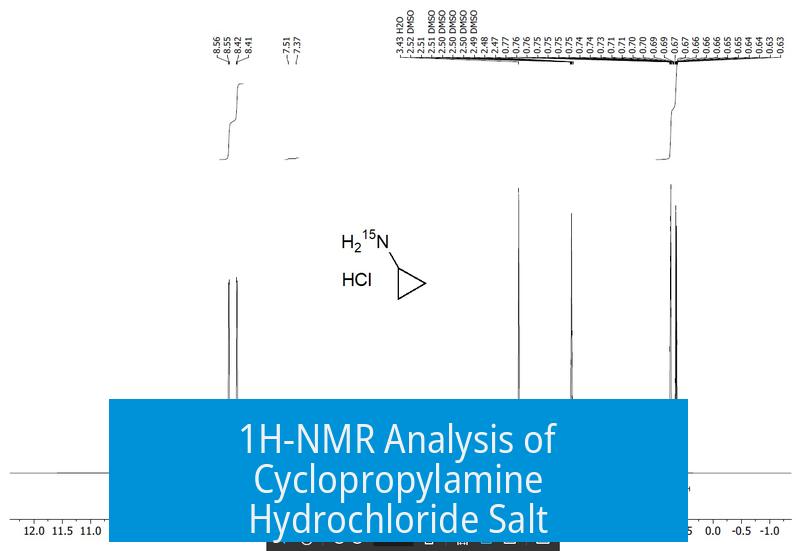
The 1H-NMR spectrum of cyclopropylamine hydrochloride salt exhibits a characteristic triplet near 7.4 ppm, assigned to ammonium chloride (NH4Cl) protons. This signal is influenced by the electron-poor nitrogen environment, causing notable deshielding and resulting in the downfield chemical shift.
Ammonium Chloride Signal and Chemical Shift
The prominent 1:1:1 triplet at approximately 7.4 ppm corresponds to the NH4Cl proton environment. This downfield position is due to the proton being alpha to an electron-deficient nitrogen, which reduces electron density around the proton, leading to significant deshielding and a shifted resonance.
Splitting Patterns: Ring and Coupling Effects
The triplet pattern arises not from coupling with the nitrogen nucleus (14N), but rather from the cyclopropane ring structure. Specifically, the methine proton couples with two syn diastereotopic methylene protons, producing the observed triplet splitting.
The trans methylene protons exhibit a Karplus dihedral angle near 80-90°, associated with very small coupling constants. These couplings remain unresolved in the standard 1H-NMR spectrum, which explains why only the syn coupling is visible.
Methine Proton Signal and Overlapping Peaks
The methine proton signal is typically not detected directly. It likely overlaps with the DMSO solvent peak, obscuring its observation. Advanced two-dimensional techniques, such as HQSC (Heteronuclear Single Quantum Coherence), are recommended to confirm the presence of this proton beneath the solvent signal.
Isotopic Labeling for Signal Confirmation
Preparation of a 15N-labeled cyclopropylamine hydrochloride analog confirms the NH4Cl signals near 7.5 ppm, solidifying the assignment of the ammonium chloride triplet in the spectrum.
Signal Integration Considerations
- Exact quantitative integration of the triplet assigned to NH4Cl protons is discouraged, as it may not precisely correspond to one proton.
- Overlapping signals and complex coupling patterns complicate integration accuracy.
Summary of Key Points
- Strong triplet at ~7.4 ppm represents ammonium chloride protons due to deshielding by electron-poor nitrogen.
- Triplet arises from coupling of methine proton with two syn diastereotopic methylene protons, not 14N coupling.
- Trans proton couplings have near 90° Karplus angles, causing very weak, often unobserved couplings.
- Methine proton signals may be hidden under the DMSO solvent peak, requiring 2D NMR for confirmation.
- 15N isotopic labeling validates ammonium chloride peak assignments.
- Integration of ammonium chloride triplet should be approached cautiously.
Unlocking the Secrets of 1H-NMR for Cyclopropylamine HCl Salt
What does the 1H-NMR spectrum of cyclopropylamine hydrochloride salt actually tell us? The key takeaway is that the signature triplet near 7.4 ppm is the unmistakable sign of ammonium chloride in the sample. This single insight sparks a fascinating story about protons, rings, and the mysteries hiding right under our NMR needles.
So, let’s unpack this spectral puzzle with a dash of chemistry and a sprinkle of humor.
Why Does Ammonium Chloride Appear as a Triplet at 7.4 ppm?
First off, the 1:1:1 triplet at around 7.4 ppm isn’t just a random bump. It’s the ammonium chloride signal making a bold statement. Why so downfield, you ask? It’s the proximity to an electron-poor nitrogen atom. See, protons alpha to these nitrogens experience heavy deshielding, which means they resonate at a lower field, pushing their peaks downfield in the spectrum.
Imagine these protons like people standing next to a huge, intimidating electronegative neighbor. They get all the attention, literally, and feel the “magnetic pressure.” Thus, their NMR signals shift downfield.
The Triplet’s Unique Splitting Explained
The multiplicity here isn’t just for show. It’s neat chemistry at play, driven by the cyclopropane ring structure. The methine proton couples specifically to the two syn diastereotopic methylene protons inside that strained ring. This coupling produces the distinctive triplet pattern, not coupling to the 14N nucleus, which is often the usual suspect.
And about those trans protons? Here’s a fun fact: their Karplus dihedral angle is near 80-90°. This angle results in almost silent coupling constants—so tiny the NMR machine can’t detect them.
Why Can’t We See the Methine Proton Clearly?
If you’re scratching your head wondering why the methine proton plays hide and seek, the culprit is the solvent, DMSO. The methine proton peak typically hides under the broad DMSO signal, making it nearly invisible in standard 1H-NMR runs.
Advanced techniques, like HQSC (Heteronuclear Single Quantum Coherence spectroscopy), become the NMR detective’s magnifying glass, helping chemists confirm if this peak is lurking beneath the DMSO veil.
Beware When Integrating Peaks!
We all love precise numbers, but here’s a caution: avoid obsessing over quantitatively integrating this triplet to exactly one proton. Complex coupling and overlapping signals mean the integration might mislead you, like trying to count fish in a bubbling stream.
How Isotopic Labeling Confirms the Puzzle Pieces
One of the most elegant confirmations comes from isotopic magic. By substituting the nitrogen with its 15N isotope variant, researchers confirmed that those signals near 7.5 ppm genuinely correspond to ammonium chloride.
This isotopic tweak removes any doubt, transforming speculation into solid evidence—a real “aha” moment for any NMR enthusiast.
Putting It All Together: Practical Tips and Takeaways
- Look for the triplet at ~7.4 ppm: This is your ammonium chloride hallmark in cyclopropylamine HCl salt’s 1H-NMR spectrum.
- Understand coupling complexity: The cyclopropane ring and diastereotopic protons create unique splitting, which is more than simple spin-spin coupling.
- Beware overlapping signals: DMSO solvent peaks can mask important protons like the methine. Use techniques like HQSC to resolve them.
- Don’t rely only on peak integration: Spectral overlap and subtle couplings can distort quantitative proton counts.
- Confirm with isotopic labeling: If you’re unsure about assignments, 15N labeling offers definite proof of ammonium chloride signals.
Why Does This Matter to You?
If you’re a chemist, student, or curious molecule wrangler, recognizing these spectral nuances can save you time and frustration. Instead of guessing why a peak behaves a certain way, you’ll confidently interpret spectra, identify contaminants like ammonium chloride, and understand the delicate dance of protons in strained rings.
This knowledge aids synthesis confirmation, purity assessments, and structural elucidation. It is like having a backstage pass to your molecule’s performance.
More Than Just Chemistry: The Story Behind the Spectrum
The 1H-NMR spectrum here tells a story of molecular intimacy and electronic environments. Proton signals feel the pull or push of neighboring atoms, leading to dramatic shifts and splits. The cyclopropane ring introduces diastereotopic protons that couple in distinctive ways. And then, the ammonium chloride makes its presence loudly known with a signature triplet.
It’s less sterile data and more molecular gossip—who’s whispering to whom, who’s hiding, and who’s shining under the spotlight.
As instrumentation evolves, tools like HQSC and isotopic labeling become invaluable allies, peeling back layers of spectral complexity. They help chemists not just observe but truly understand molecules like cyclopropylamine hydrochloride salt.
Curious to see this spectrum in action? Check out the reference spectrum of cyclopropylamine hydrochloride 15N labeled 1H-NMR for a real-world peek into this chemical story.
Next time you run an NMR with cyclopropylamine HCl salt, you’ll know exactly where to look and what to think. Now isn’t that a triplet well worth the journey?
What causes the triplet signal at around 7.4 ppm in the 1H-NMR of cyclopropylamine HCl salt?
The triplet at ~7.4 ppm is due to ammonium chloride (NH4Cl) in the sample. It results from coupling patterns of the ammonium protons.
Why does the proton signal near 7.4 ppm appear downfield in the spectrum?
It is alpha to an electron-poor nitrogen, causing significant deshielding that shifts the proton resonance downfield.
How does the cyclopropane ring influence the observed splitting pattern?
The methine proton couples with the two syn diastereotopic methylene protons. This interaction creates the characteristic 1:1:1 triplet instead of coupling to 14N.
Why might the methine proton signal be missing or hard to detect?
The methine proton often hides beneath the DMSO solvent peak. Advanced methods like HQSC can help reveal it by separating overlapping signals.
How can isotopic labeling assist in confirming peak assignments?
Using a 15N-labeled variant confirms that signals near 7.5 ppm correspond to NH4Cl, clarifying ambiguous spectral features.



Leave a Comment