Are 1,4 Disubstituted Cyclohexanes Always Achiral?

1,4 disubstituted cyclohexanes are generally achiral due to the presence of a plane of symmetry in their molecular structure, regardless of the substituents involved. This symmetry element negates any potential chirality that might arise from the individual stereocenters.
Symmetry and Chirality in 1,4 Disubstituted Cyclohexanes
The primary reason for the achirality of 1,4 disubstituted cyclohexanes lies in their overall molecular symmetry. These molecules usually possess a plane of symmetry or an improper rotation axis (S1-axis), which means that even if the substituents create stereocenters at positions 1 and 4, the molecule as a whole is superimposable on its mirror image.
- Cis and trans isomers of 1,4 disubstituted cyclohexanes both feature symmetry planes.
- Neither cis nor trans forms exhibit chirality despite having stereocenters.
- For example, 1,4-dimethylcyclohexane exists as cis and trans but remains achiral due to this symmetry.
Conformation and Achirality
In their common chair conformations, 1,4 disubstituted cyclohexanes maintain achirality. The trans isomer predominantly adopts a diequatorial conformation at equilibrium, which reinforces the molecular symmetry.
This conformational behavior contributes to the absence of optical activity since no chiral axis or center persists in the preferred conformer.
Diastereomers and Achirality

The cis and trans 1,4 disubstituted cyclohexanes are stereoisomers, more specifically diastereomers, because they differ in spatial arrangement, but each stereoisomer is individually achiral.
This distinction clarifies that diastereomerism does not necessarily imply chirality; molecules can differ stereochemically yet lack optical activity if symmetry elements exist.
Comparison with Other Positional Isomers
The 1,4 disubstituted cyclohexanes differ from 1,2- or 1,3-disubstituted isomers, which may be chiral due to the absence of symmetry planes.
The position of substituents critically influences molecular symmetry and thus chirality.
Conceptual Visualization
An analogy treats the cyclohexane ring like a double bond, with substituents in cis (same side) or trans (opposite sides) configurations. Though such arrangements influence stereochemistry, the inherent symmetry in the 1,4 positions renders these molecules achiral.
Key Takeaways

- 1,4 disubstituted cyclohexanes possess a plane of symmetry, making them achiral.
- Cis and trans isomers are diastereomers but individually lack chirality.
- The diequatorial chair form of trans isomers predominates, maintaining achirality.
- Other substitution patterns (1,2- or 1,3-) may result in chiral cyclohexanes due to reduced symmetry.
- Molecular symmetry plays a decisive role in the chirality of substituted cyclohexanes.
Are 1,4 Disubstituted Cyclohexanes Always Achiral?
Yes, 1,4 disubstituted cyclohexanes are always achiral due to their inherent symmetry. The magic trick here is the presence of a plane of symmetry in the molecule, which cancels out any chance of chirality. Even if you look closely and imagine each substituted carbon as a “chiral center,” the molecule overall remains achiral because the spatial arrangement balances itself perfectly.
Let’s break down why this happens and why it matters for anyone diving into organic chemistry or molecular design.
Why Symmetry Rules the Chirality Game
In the world of molecules, chirality means a molecule is like your left and right hands—non-superimposable mirror images. But imagine a mirror in the middle of 1,4 disubstituted cyclohexane—this mirror reflects one side onto the other perfectly. This “plane of symmetry” means the molecule and its mirror image are essentially the same, making it achiral.
For example, in 1,4-dimethylcyclohexane, both cis and trans forms exist, but neither is chiral. Why? They have a plane of symmetry in respective conformations. This key symmetry cancels out any “handedness.” The molecule looks identical no matter how you turn it.
“1,4-disubstituted cyclohexanes are always achiral. The disubstituted six-membered ring has a plane of symmetry, making it achiral.”
But What About Chiral Centers? Aren’t They Always Chiral?

Great question! The two substituents are attached to carbons that might appear chiral if you look individually. However, the entire molecule’s overall symmetry destroys that chirality on a macro scale. Essentially, the molecule has an S1 axis of symmetry and notable “2,6-chain symmetry” that offsets possible chiral centers.
This means even if you call both substituted carbons “chiral,” the molecule refuses to be chiral as a whole. Chemistry’s way of saying, “You can’t fool me.”
Can Diastereomers Exist Here?
Here’s where some confusion creeps in. People ask, “If 1,4-disubstituted cyclohexanes are achiral, how come there are diastereomers?” It’s an excellent point, so let’s clarify.
Diastereomers are stereoisomers that are not mirror images, and yes, they can exist for 1,4 disubstituted cyclohexanes. For example, the cis and trans isomers are diastereomers of each other. Both forms are achiral but different enough spatially to qualify as diastereomers. So achirality does not exclude isomerism other than enantiomerism.
Imagine a Cyclohexane Like a Double Bond
One helpful way to visualize this is to think about the substituents like on a double bond, with cis and trans orientations. The substituents on a 1,4 cyclohexane can be on the same side (cis) or opposite sides (trans), analogous to cis/trans isomers of alkenes. The differences lead to distinct diastereomers but don’t break symmetry enough to spark chirality.
This visualization helps because it shifts the perspective from complicated cyclohexane rings to something more familiar: double bonds. And we know those cis/trans isomers from basic chemistry class.
Chair Conformers: Still Achiral, But More Comfortable
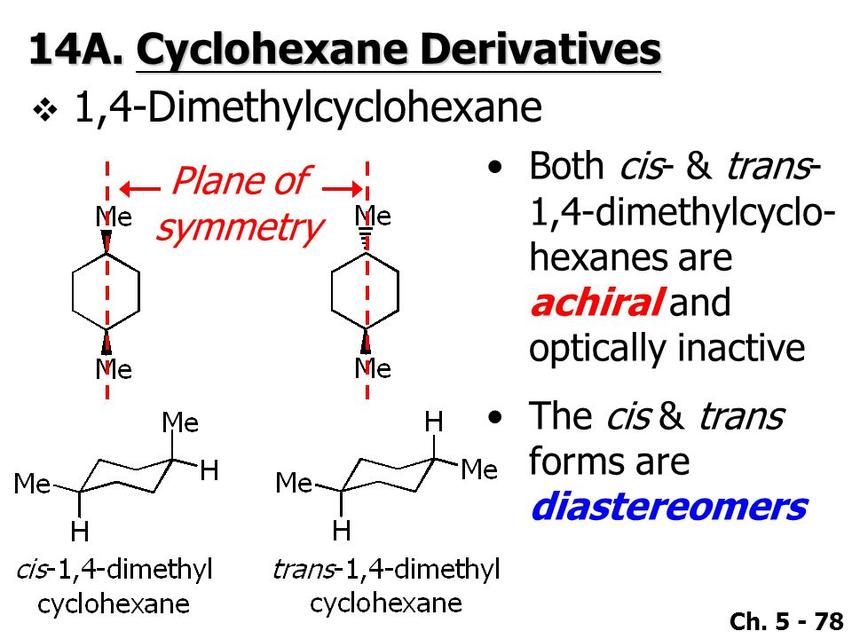
Another fascinating fact is that, in cyclohexanes, the chair conformer dominates. It turns out that all chair conformers of 1,4 disubstituted cyclohexane are achiral, especially the diequatorial conformer of the trans isomer, which is usually the most stable and predominant at equilibrium.
Stability meets symmetry, and chirality takes a backseat.
What Happens If the Substituents Are Different?
Here’s a fun twist: if the two substituents on carbons 1 and 4 are different (not identical groups), the absolute symmetry gets broken, and the molecule might become chiral. This is crucial because the symmetry that guaranteed achirality relies on identical substituents.
So, the statement “1,4 disubstituted cyclohexanes are always achiral” strictly applies to molecules with identical substituents at those positions. Changing the game with distinct groups opens the door to chirality.
Summary and Practical Takeaways
- 1,4 Disubstituted cyclohexanes with identical substituents are always achiral.
- This achirality arises because of their plane of symmetry and overall spatial arrangement.
- They can have diastereomers (cis and trans), but these are not mirror images, so they are achiral diastereomers.
- Think of substituents like on a double bond. Their relative positions define isomers but not chirality.
- Chair conformers remain achiral and usually favor the diequatorial trans form.
- Introducing different substituents at 1 and 4 can produce chirality by disrupting symmetry.
In conclusion, the beauty of 1,4 disubstituted cyclohexanes is in their symmetry and the way it rules out chirality under specific conditions. This knowledge is critical for students, chemists, and anyone designing molecules where stereochemistry matters. It helps predict behavior, chemical reactivity, and even properties in fields like drug design and materials science.
Next time you sketch out a 1,4 disubstituted cyclohexane, remember: the molecule’s mirror is kinder than you think—it’s perfectly symmetrical and achiral unless you mess with those substituents!
Are all 1,4 disubstituted cyclohexanes achiral?
Yes, they are generally achiral. This is due to the plane of symmetry in the molecule. Even if centers look chiral, overall symmetry prevents chirality.
Do cis and trans isomers of 1,4 disubstituted cyclohexanes show chirality?
No. Both cis and trans isomers are achiral. Each has a plane of symmetry, keeping the molecule free from chirality.
Can 1,4 disubstituted cyclohexanes have chiral centers yet be achiral?
Yes. Even if centers are defined as chiral, the molecule can be achiral because of symmetry elements like S1 axes or mirror planes.
How does the chair conformation affect chirality in 1,4 disubstituted cyclohexanes?
All chair conformers remain achiral. The trans isomer’s diequatorial conformer predominates but does not introduce chirality.
Are the cis and trans forms of 1,4 disubstituted cyclohexanes diastereomers?
Yes, they are diastereomers of each other. They are both achiral but differ in spatial arrangement, making them distinct stereoisomers.



Leave a Comment