Are Diastereomers Optical Isomers? Understanding the Relationship
Diastereomers are not always optical isomers; they may or may not exhibit optical activity, and if they do, their direction of rotating plane-polarized light is not necessarily opposite.
Optical Activity and Its Meaning
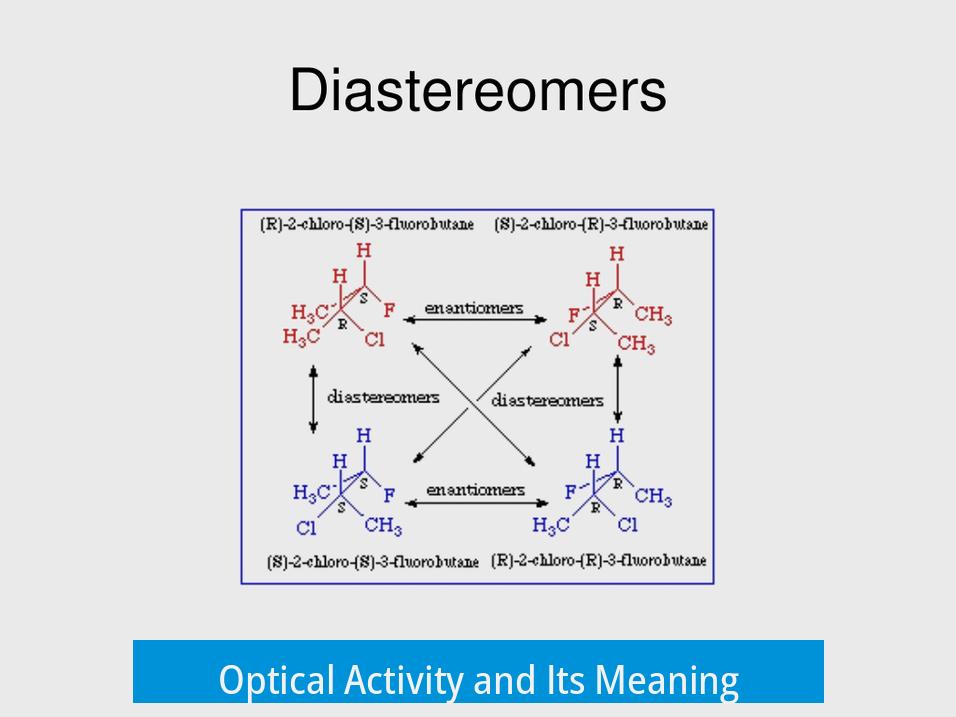
Optical activity is a molecule’s ability to rotate plane-polarized light. A compound that affects polarized light is called optically active. This property depends on the molecule’s stereochemistry, especially the arrangement of atoms in space.
Enantiomers as Optical Isomers
Enantiomers are a specific type of stereoisomers that are non-superimposable mirror images. They exhibit optical activity in a well-defined way: one enantiomer rotates plane-polarized light clockwise (dextrorotary), and the other rotates it counterclockwise (levorotary) with the same magnitude but in opposite directions. This reciprocal relationship is the hallmark of optical isomers commonly recognized in chemistry.
Diastereomers and Their Optical Behavior
Diastereomers, unlike enantiomers, are stereoisomers that are not mirror images. They may differ in some but not all stereocenters. Crucially, diastereomers are not required to be optically active. Some diastereomers show optical activity while others do not, depending on their specific spatial arrangements.
When diastereomers are optically active, their rotation of plane-polarized light does not have a simple, opposite relationship like enantiomers. They can rotate light in the same direction or different magnitudes. This behavior means diastereomers are not generally considered optical isomers under the strict definition tied to enantiomers.
Terminology and IUPAC Recommendations
The International Union of Pure and Applied Chemistry (IUPAC) discourages using the term “optical isomers” broadly because optical activity can be complex. They recommend precise language when discussing stereoisomers and optical properties. This highlights the importance of distinguishing enantiomers from diastereomers in describing their optical behaviors.
Summary of Key Points
- Diastereomers may or may not be optically active.
- Optically active diastereomers do not necessarily rotate polarized light in opposite directions.
- Enantiomers are optical isomers by definition because they rotate light equally but oppositely.
- IUPAC advises precise terminology, avoiding broad or ambiguous use of “optical isomers.”
Are diastereomers always optically active?
No, diastereomers are not always optically active. Some diastereomers can be inactive, while others may show optical activity depending on their structure.
If diastereomers are optically active, do they rotate light in opposite directions?
Not necessarily. Diastereomers that are optically active can rotate plane-polarized light in the same or different directions. Their rotation does not have a fixed relationship like enantiomers.
How do optical isomers differ from diastereomers in terms of light rotation?
Optical isomers, specifically enantiomers, rotate plane-polarized light in opposite directions with equal magnitude. Diastereomers do not follow this rule and may vary in rotation behavior.
Can diastereomers be considered optical isomers?
Diastereomers can be optical isomers if they rotate plane-polarized light. However, they do not fit the strict definition of optical isomers commonly used for enantiomers.
Why is the term “optical isomers” discouraged by IUPAC for diastereomers?
IUPAC prefers precise terms because “optical isomers” is often used only for enantiomers. Using it for diastereomers can cause confusion since diastereomers may differ in optical activity and rotation behavior.




Leave a Comment