Does Protonated Acetone Have Resonance Structures?

Protonated acetone generally does not exhibit resonance structures when protonation occurs on the oxygen atom, as the carbonyl carbon maintains a full octet and the protonation stabilizes the positive charge on oxygen without delocalization.
Protonation Site in Acetone
Acetone contains a carbonyl group, where the oxygen atom is more electronegative than carbon. In acidic conditions, protonation typically happens on the oxygen atom rather than the carbon. This is because oxygen’s lone pairs can stabilize the added proton effectively, maintaining a full octet.
Octet Considerations After Protonation
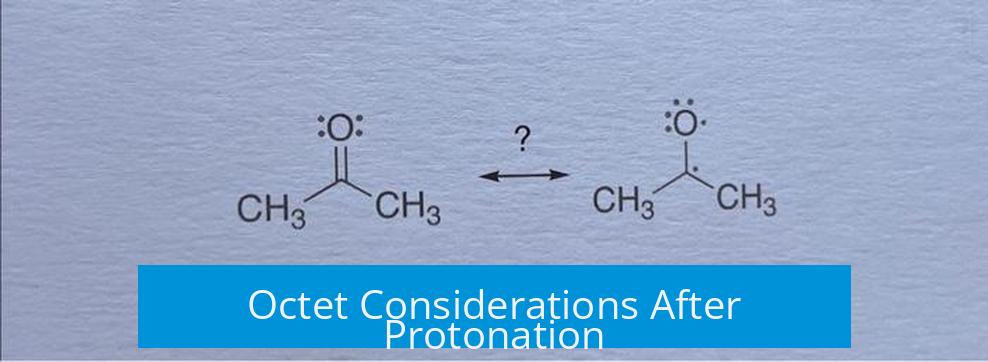
When acetone’s oxygen is protonated, the oxygen atom retains its octet by forming a bond with a proton. The carbonyl carbon also satisfies the octet rule, bonded to oxygen and three other substituents. Thus, no atom exceeds or lacks an octet in the protonated form.
Why Protonation Does Not Lead to Resonance in this Case
- Protonation on oxygen leads to a positively charged oxonium ion.
- This positive charge is localized primarily on oxygen.
- The carbonyl double bond loses its classic resonance with the carbonyl carbon, as the oxygen’s lone pairs are now engaged in bonding with the proton.
- The carbon atom’s octet remains intact without a need for electron delocalization to stabilize charge.
In typical acetone, resonance arises from delocalization between the oxygen and carbonyl carbon. However, protonation locks the oxygen’s electrons in the bond to the proton, reducing resonance possibilities.
Summary Table: Protonation and Resonance in Acetone

| Feature | Protonated Acetone | Reasoning |
|---|---|---|
| Protonation Site | Oxygen atom | More electronegative; stabilizes positive charge |
| Carbon Octet | Complete | Maintains four bonds; no need for resonance |
| Resonance Structures | Absent or minimal | Positive charge localized on oxygen; no double bond delocalization |
Key Takeaways
- Protonation in acetone mostly occurs at the oxygen atom due to electronegativity.
- Both oxygen and carbon atoms preserve their octets in protonated acetone.
- The positive charge from protonation localizes on oxygen, preventing resonance with carbon.
- Carbon maintains its octet without electron delocalization.
Does Protonated Acetone Have Resonance Structures? Unpacking Octets and Protonation
Short answer: Protonated acetone usually does not have resonance structures involving the protonation site because the carbon’s octet is already satisfied, and protonation occurs at oxygen, which keeps its octet intact. But wait—why is this so? And what about the Lewis structures puzzling you? Let’s untangle this step-by-step.
Imagine you’ve drawn acetone’s Lewis structure neatly on paper. The carbon atoms, each snug with their octet, seem content. Then the question arises—“If I protonate acetone, especially the oxygen or carbon, can I draw resonance structures?” The carbon’s octet is satisfied, so does resonating even make sense?
Where Does Protonation Happen? Oxygen Wins the Popularity Contest
Protonation means adding a proton (H+) somewhere on the acetone molecule. The first, and crucial, detail: it almost always happens on oxygen, not carbon. Oxygen beats carbon hands down here because it is more electronegative and holds electrons tighter. This makes oxygen the preferred site for protonation.
Think of oxygen as the electron-lover who throws a party for that H+, whereas carbon politely declines, already full with its electrons (octet satisfied, remember?). So, acknowledging that oxygen is where the H+ buddies up is essential before thinking about resonance.
Does protonating carbon make sense? Usually not. Carbon’s octet is intact and stable. Also, a proton on carbon would create an unusual positive charge and disturb that balance. In contrast, protonated oxygen keeps things neat and tidy.
Octets in Play: Carbon’s Satisfaction vs. Oxygen’s New Dance Partner
When you protonate oxygen in acetone, what happens to the octet rule? Both carbon and oxygen strive to keep their octets intact. Carbon is happily bonded to four atoms, fulfilling its octet. When oxygen accepts the proton, it forms three bonds and carries a positive charge, but its octet remains complete too.
This careful preservation of octets explains why protonated acetone doesn’t lend itself to multiple resonance forms arising from the protonation site. There’s no electron “sloshing” back and forth like in typical resonance scenarios.
Does this mean no resonance at all? Not exactly. The broader acetone molecule has its own resonance-related chemistry in some contexts (like enolate formation), but protonated acetone’s protonation spot is straightforward—oxygen locks down the proton, keeping octets satisfied, and there’s no resonance involving the protonated oxygen-carbon framework.
Resonance Structures: Why They Don’t Show Up in Protonated Acetone
Resonance structures usually crop up when there are alternative ways to place double bonds or charges without moving atoms. They help represent electron delocalization that stabilizes molecules.
In protonated acetone, the positive charge from the added proton localizes on the oxygen, which already shares electrons through its bonds. Since carbon’s octet remains untouched and the oxygen’s new bond completes its octet, there’s no wiggle room for moving bonds around. No resonance forms exist that shuffle electrons between carbon and oxygen in this protonated state without breaking the octet rule or moving atoms.
Imagine resonance as a dance where one partner can switch positions without stepping on the toes of the other dancers. Here, oxygen’s proton hold is firm and fixed, so no dance moves—no resonance.
Practical Tip: Trust the Electronegativity and Octet Rule as Guides
If you struggle whether to protonate carbon or oxygen, always bet on oxygen. Its electronegativity and ability to stabilize the positive charge by completing its octet make it the prime candidate. This approach clears confusion and aligns with experimental chemistry.
Also, be mindful about drawing resonance structures: check if electrons move without violating octet rules or atom positions. For protonated acetone, those checks tell us resonance structures don’t exist around the protonation site.
Summary Table: Quick Comparison of Sites and Resonance
| Aspect | Oxygen Protonation | Carbon Protonation |
|---|---|---|
| Likelihood | High (oxygen is electronegative) | Very low (carbon octet satisfied) |
| Octet Rule | Octet maintained | Octet satisfied but protonation is unusual |
| Resonance Structures | No resonance involving protonation site | No practical resonance possible |
| Charge Localization | Positive charge on oxygen | Unstable positive charge on carbon |
Personal Take and Real-World Chemistry
In lab practice and theory, chemists never consider protonating carbon in acetone just for kicks. It’s oxygen’s playground, and protonated acetone’s positive charge mainly sits right there, like a polite guest who doesn’t move around too much.
This understanding also simplifies reaction mechanisms. When acids interact with ketones like acetone, we focus on the protonated oxygen intermediate. Resonance structures for this protonated form? Not needed; the molecule prefers straightforward stability.
Next time you wonder about Lewis structures and resonance, keep this scenario in mind: sometimes simplicity rules. Not every protonation or positive charge leads to resonance. Stability and electron rules guide these decisions.
Questions to Ponder
- Could protonated acetone act differently in exotic environments where carbon gets involved in protonation?
- How does resonance play a role in related compounds like enols or enolates derived from acetone?
- What experimental evidence confirms oxygen protonation over carbon in acetone?
Exploring these questions furthers your grasp on organic chemistry’s neat yet complex dance of electrons.
In summary, protonated acetone keeps its carbon octet satisfied and chooses oxygen as protonation site, which doesn’t produce resonance forms involving the protonation spot. That’s the chemistry truth you can bet on!
Does protonated acetone have resonance structures?
Protonated acetone typically does not show resonance structures focused on the carbon. The protonation occurs on oxygen, which keeps the octet intact. Resonance is not a major factor in this protonated form.
Why is oxygen protonated in acetone instead of carbon?
Oxygen is more electronegative than carbon. It can better stabilize the positive charge after protonation. Protonating carbon would disrupt its octet and is less favorable.
Can the oxygen in protonated acetone violate the octet rule?
No. When oxygen is protonated, it still maintains an octet. Protonation adds a hydrogen, but oxygen shares electrons to keep eight in its valence shell.
If the carbon octet is satisfied, why consider resonance?
Resonance involves electron delocalization. Since protonation occurs on oxygen and carbon keeps an octet, resonance structures from carbon are not generally drawn here.
Is it possible to draw resonance structures involving protonated oxygen in acetone?
While protonated oxygen can influence electron density, classical resonance structures for protonated acetone mainly emphasize protonation site rather than resonance. The oxygen maintains its octet with no major resonance shifts.



Leave a Comment