Which Carbocation is More Stable?
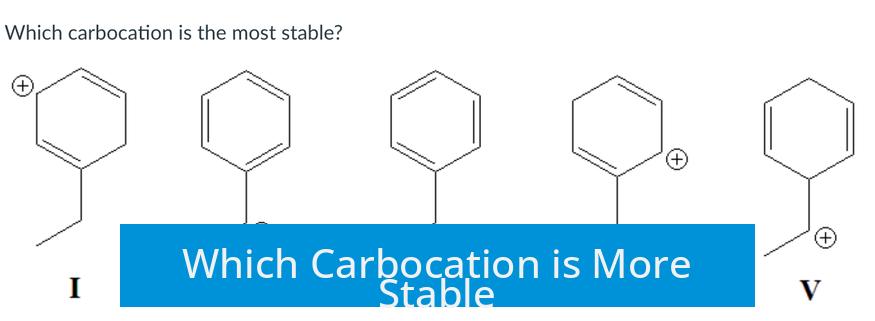
The benzylic carbocation is more stable than the tertiary carbocation due to resonance stabilization. Unlike tertiary carbocations, which rely primarily on alkyl group electron donation, benzylic carbocations benefit from resonance and aromaticity that delocalize the positive charge.
Stability Order of Carbocations
The general stability order reflects the influence of resonance and alkyl substitution:
- Benzylic (most stable)
- Allylic
- Tertiary
- Secondary
- Primary (least stable)
This order emphasizes that resonance effects and conjugation play a larger role than simply the number of alkyl groups attached.
Why Benzylic Carbocations Are Most Stable
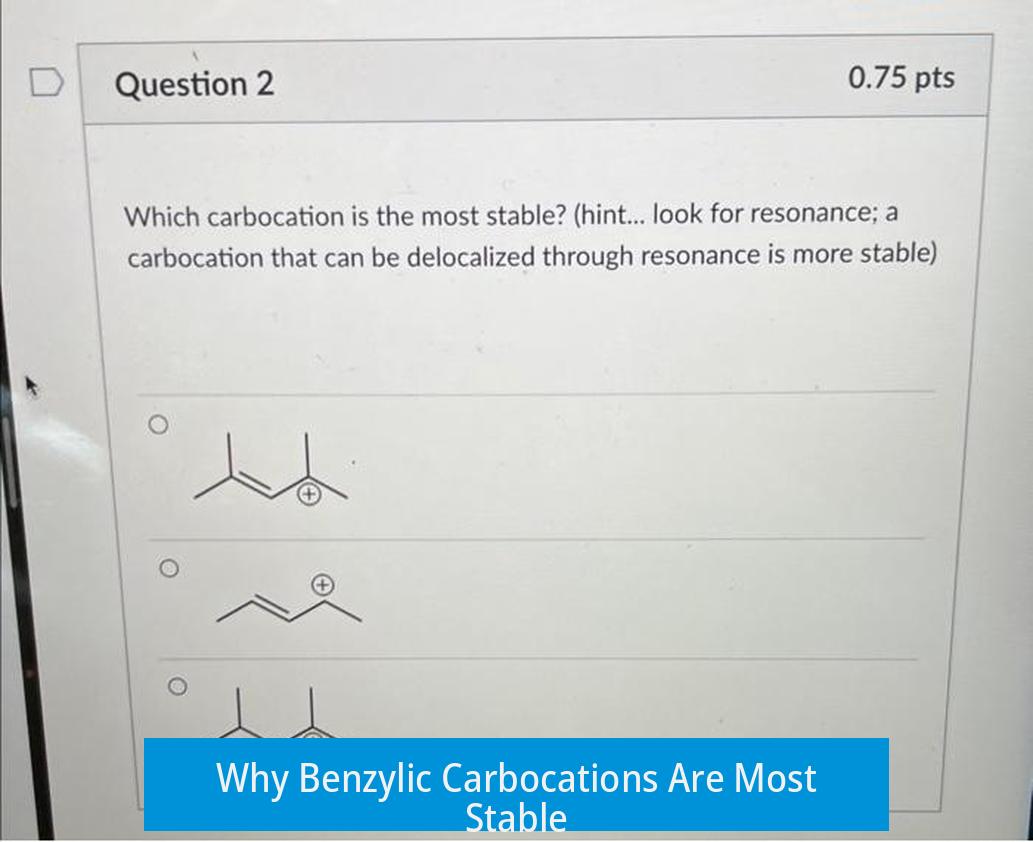
Benzylic carbocations are stabilized because the positive charge can delocalize into the aromatic ring via resonance. This delocalization spreads out the charge over several atoms, lowering the system’s energy.
Drawing resonance structures for the benzylic carbocation shows multiple contributors, highlighting the extensive charge distribution. This resonance is absent in purely alkyl-substituted carbocations.
Comparison with Tertiary Carbocations
Tertiary carbocations gain stability mainly from alkyl groups donating electron density through hyperconjugation and inductive effects. While these effects are significant, they cannot match the resonance stabilization of benzylic carbocations.
Textbook analyses show that:
“Because of resonance stability, a primary or benzylic carbocation is about as stable as a secondary alkyl carbocation, and a secondary allylic or benzylic carbocation is about as stable as a tertiary alkyl carbocation.”
This highlights that resonance can elevate the relative stability of a carbocation beyond what alkyl substitution alone achieves.
Key Points to Remember
- Benzylic carbocations are more stable than tertiary carbocations due to resonance and aromaticity.
- Resonance delocalizes positive charge in benzylic carbocations, stabilizing them effectively.
- Tertiary carbocations rely on alkyl group stabilization but lack resonance effects.
- Overall stability ranking: benzylic > allylic > tertiary > secondary > primary.
- Resonance effects can outweigh multiple alkyl substituents in carbocation stability.
Which carbocation is generally the most stable?
Benzylic carbocations are usually the most stable. This is due to resonance and aromaticity, which help spread out the positive charge over the ring structure.
Why is a benzylic carbocation more stable than a tertiary carbocation?
Benzylic carbocations gain extra stability from resonance. The positive charge is delocalized across the aromatic ring. Tertiary carbocations rely only on alkyl group stabilization, which is less effective.
How does resonance affect carbocation stability?
Resonance allows the positive charge to be shared over multiple atoms. This lowers the energy of the carbocation, making it more stable compared to carbocations without resonance.
What is the stability order of common carbocations?
- Benzylic (due to resonance and aromaticity)
- Allylic
- Tertiary
- Secondary
- Primary
Can a primary carbocation be as stable as a secondary one?
If the primary carbocation is benzylic, yes. Resonance makes some primary carbocations nearly as stable as secondary alkyl carbocations.
How do alkyl groups stabilize carbocations compared to resonance?
Alkyl groups stabilize by donating electron density through inductive effects. This is weaker than resonance stabilization, which delocalizes the charge over several atoms.


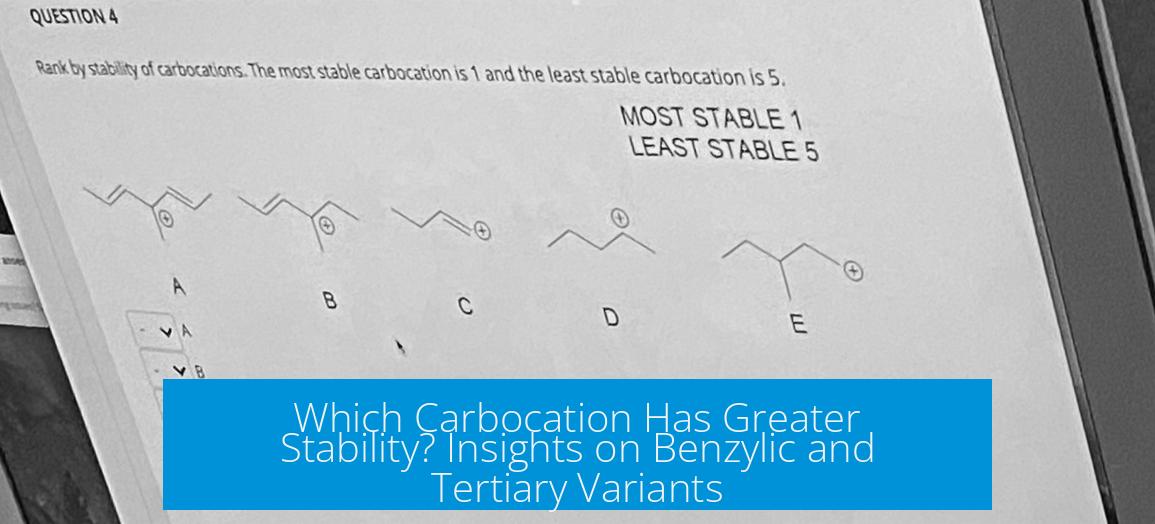


Leave a Comment