Why is the -COOH (Carboxyl Group) Polar?
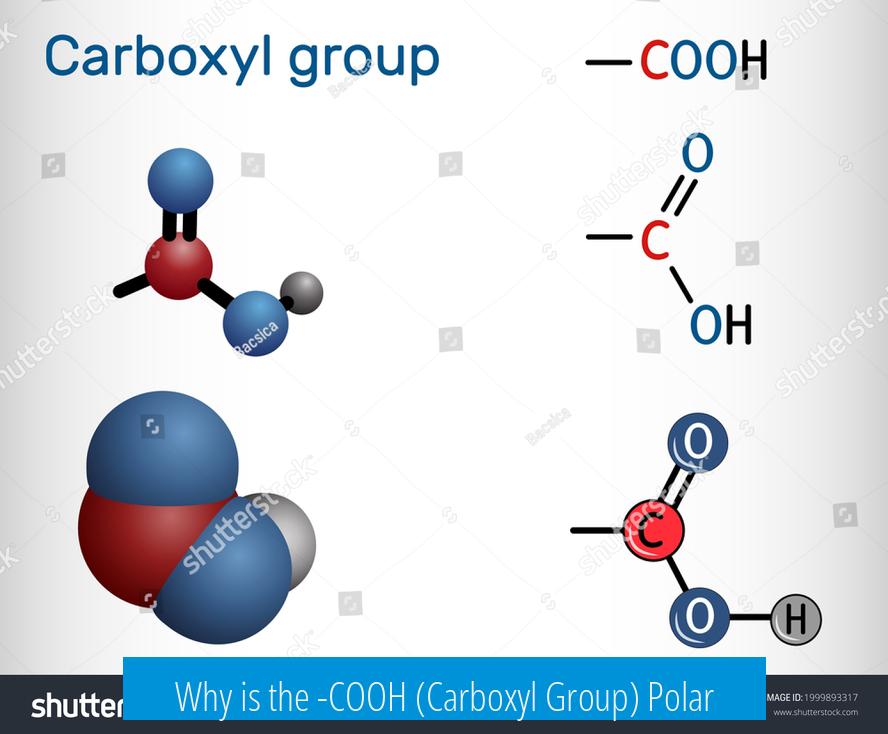
The -COOH group is polar due to the presence of polar bonds whose dipole moments do not cancel, combined with electron delocalization that enhances the group’s overall polarity. This polarity arises mainly because of the distinct electronegativities of the atoms involved and the specific bonding structure within the group.
Bond Polarity Within the -COOH Group

The carboxyl group contains two key polar bonds: the carbon-oxygen double bond (C=O) and the oxygen-hydrogen bond (O-H). The C=O bond is moderately polar due to oxygen’s higher electronegativity compared to carbon. The O-H bond is very polar because of the large electronegativity difference between oxygen and hydrogen, creating a strong dipole.
- C=O bond: Moderately polar.
- O-H bond: Highly polar, enabling hydrogen bonding.
These two bonds create bond dipole moments in different directions. When mapped as vectors, they do not cancel but instead add up, leading to a net dipole moment. This net dipole confirms the polarity of the entire -COOH group.
Electron Delocalization Enhances Polarity
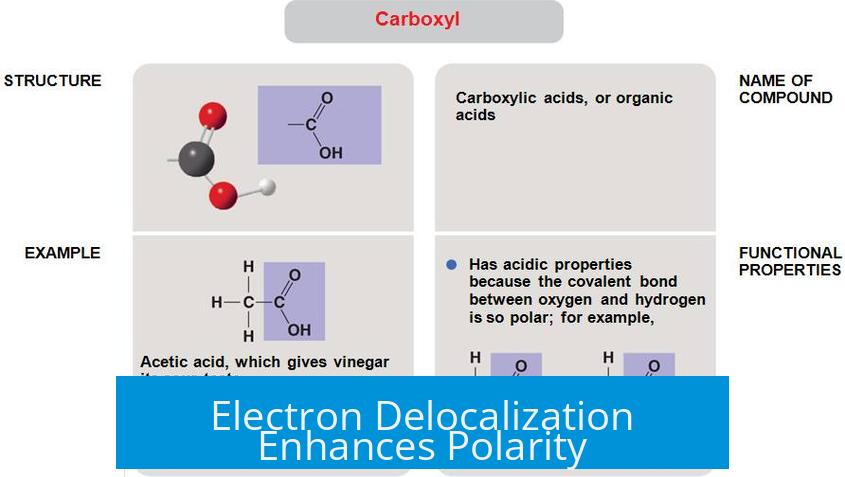
Electrons in the carboxyl group are delocalized between the two oxygen atoms. This sharing and resonance stabilize the group and amplify the polarity.
- Increases hydrogen bond donating ability of the -OH group.
- Enhances hydrogen bond accepting ability of the C=O group.
This delocalization strengthens the intermolecular interactions, making the carboxyl group more polar than either a simple hydroxyl or carbonyl group alone.
Distinguishing Polar Bonds from Polar Molecules
It is important to note that polar bonds alone do not guarantee a polar molecule. The geometry and arrangement of bonds determine whether dipole moments cancel out.
In the case of -COOH, its geometry ensures the bond dipoles reinforce rather than negate each other, yielding an overall polar group.
Example of Nonpolar Contrast
Triglycerides contain C=O bonds but lack the -OH group. They also have many nonpolar C-C and C-H bonds. This combination results in a largely nonpolar molecule. By contrast, the carboxyl group’s polar O-H and C=O bonds produce significant polarity.
Key Takeaways
- -COOH is polar because its bond dipoles from C=O and O-H do not cancel.
- The O-H bond is strongly polar and engages in hydrogen bonding.
- Electron delocalization in the group increases polarity further.
- Polar bonds must be arranged to avoid dipole cancellation for molecular polarity.
- Nonpolar molecules may contain polar bonds but can be overall nonpolar due to geometry and other bonds.
Why is -COOH (Carboxyl Group) Polar? Unlocking the Mystery!
Ever paused to wonder why the humble -COOH group, known as the carboxyl group, is polar? It’s a tiny part of organic molecules but packs a punch in chemistry. The reason: its dipoles just don’t cancel out, making the whole group quite the polar protagonist. That’s the short answer, but trust me, the story behind this polarity is as fascinating as any detective tale!
Let’s dive deeper and unravel why carboxyl groups are polar, why that matters, and what sets them apart from other groups. Ready to get scientific with a smile? Here we go.
Dipoles: The Invisible Tug-of-Wars Inside -COOH
At its core, polarity means some part of the molecule hogs electrons more. The carboxyl group features two key polar bonds: the carbonyl C=O bond and the hydroxyl O-H bond.
“Basically, yes. Now draw all dipol vectors (the bond dipols) of the COOH-group. You’ll find that they don’t cancel and hence the groups must be polar overall as well.”
This means the tiny arrows representing dipoles point in different directions but never perfectly oppose or balance. The C=O bond pulls electrons toward oxygen moderately, while O-H bonds pull even harder because oxygen loves electrons and hydrogen is quite the push-over here. Together, their pull adds up, not cancels.
So the takeaway? The sum of all these polar bond dipoles results in an overall polar carboxyl group. That polarity controls how molecules behave, especially around water or in reactions.
The Shade of Polar: Bonds vs. Molecules
Let’s clear a common confusion: polar bonds don’t always make the whole molecule polar. Think of polar bonds as individuals; sometimes a crowd (the whole molecule) might cancel their opinions out.
For -COOH, those bond dipoles don’t cancel. The group is like a polar party where everyone’s opinions (dipoles) align. But zoom out to a whole molecule—say a fatty acid or amino acid—and the entire molecule’s polarity depends on other groups too.
This distinction matters because chemists often mistake polar bonds for polar molecules. Carboxyl has both, making it a reliable polar group.
Polar Powerhouses: How C=O and O-H Bonds Stack Up
Not all polar bonds are created equal. The C=O bond in -COOH is moderately polar. Picture a tug-of-war where oxygen wins but carbon puts up a fight. The O-H bond? Now that’s a heavyweight champion of polarity.
Why? Because hydrogen bonding comes into play. We recognize hydrogen bonds among O-H, F-H, and N-H as special—they’re strong dipole forces that dramatically influence properties like boiling points and solubility.
In -COOH, this means the hydroxyl part isn’t just pulling electrons; it’s ready to form hydrogen bonds. This double whammy of moderately polar C=O and highly polar O-H sets the stage for strong polarity.
Electron Delocalization: Polarity’s Secret Weapon
Here’s the real showstopper: electrons in the carboxyl group delocalize between the two oxygens. Instead of sticking around one atom, electrons spread out, stabilizing the group.
Why does this matter for polarity? Delocalization supercharges the hydrogen bond donating ability of O-H and the accepting ability of C=O. It’s like the carboxyl group is flexing both arms, grabbing and giving electrons better than any regular O-H or C=O bond.
This electron dance makes the group more polar than you’d expect from simple polar bonds. For molecules with carboxyl groups, this means stronger interactions with water and greater reactivity.
When Carboxyl Steps Out: Comparing Polar vs. Nonpolar Molecules
Not all molecules with C=O bonds are polar overall. For example, triglycerides, common fats, have plenty of C=O but miss the O-H group. They contain long C-C and C-H chains that are nonpolar beasts.
As a result, triglycerides are mostly nonpolar, floating on water with little love (insoluble). The polar C=O can’t compete against the ocean of nonpolar hydrocarbon bonds.
Here is the moral: the presence of the hydroxyl O-H in -COOH is crucial for strong polarity, giving carboxyl-containing molecules the unique ability to mingle well with water.
Practical Takeaway: Why Does Carboxyl Polarity Matter?
Polarity affects smell, taste, solubility, and how molecules react. Ever wonder why vinegar (acetic acid) dissolves so easily in water? Thank the -COOH group! It’s the polar superstar helping acetic acid mix without fuss.
- In biology, amino acids’ carboxyl groups help form proteins and maintain structure.
- In cooking, carboxyl groups influence flavor and acidity.
- In medicine, drug molecules with carboxyl groups often act differently due to polarity.
Got a molecule with a carboxyl group? Expect it to dance well in polar environments and play an active chemical role.
Summary: Carboxyl Group’s Polar Personality
| Feature | Impact on Polarity |
|---|---|
| Moderately polar C=O bond | Contributes to bond dipole but not enough alone |
| Highly polar O-H bond with hydrogen bonding | Strong hydrogen bond donor, biggest polarity booster |
| Electron delocalization between oxygens | Enhances hydrogen bond donating and accepting, increasing polarity |
| Sum of dipole vectors | Vectors add up, ensuring overall polarity of the group |
In a nutshell, the carboxyl group’s polarity stems from its unique internal bond arrangement and electron behavior – factors that help it play a starring role in chemistry and biology alike. Simple bonds? Check. Complex electron delocalization? Check. A perfect storm making -COOH polar beyond just the sum of its parts.
Next time you see a molecule with -COOH, remember—it’s got a polar personality you can’t ignore. Not bad for a tiny group, right?
Why is the carboxyl group (-COOH) considered polar?
The dipole moments of the bonds in the -COOH group do not cancel out. This makes the entire group polar, as the bond dipoles add up to form an overall dipole.
How do the polar bonds in -COOH contribute to its polarity?
The C=O bond is moderately polar. The O-H bond is highly polar and can form hydrogen bonds, which greatly increases the polarity of the carboxyl group.
What role does electron delocalization play in the polarity of -COOH?
Electrons are shared between the two oxygens, which enhances the hydrogen bonding ability of both O-H and C=O parts. This makes the entire -COOH group more polar.
Can a molecule have polar bonds but still be nonpolar? How does this relate to -COOH?
Yes. Polar bonds alone do not ensure a polar molecule. In -COOH, the bond dipoles do not cancel, so the group is polar, unlike some molecules with symmetrical polar bonds.
Why are triglycerides less polar compared to molecules with -COOH groups?
Triglycerides lack the O-H bond and have many nonpolar C-C and C-H bonds. Even though they contain C=O, their overall polarity is low, making them insoluble in water.




Leave a Comment