Acetone and Polystyrene Interaction Explained
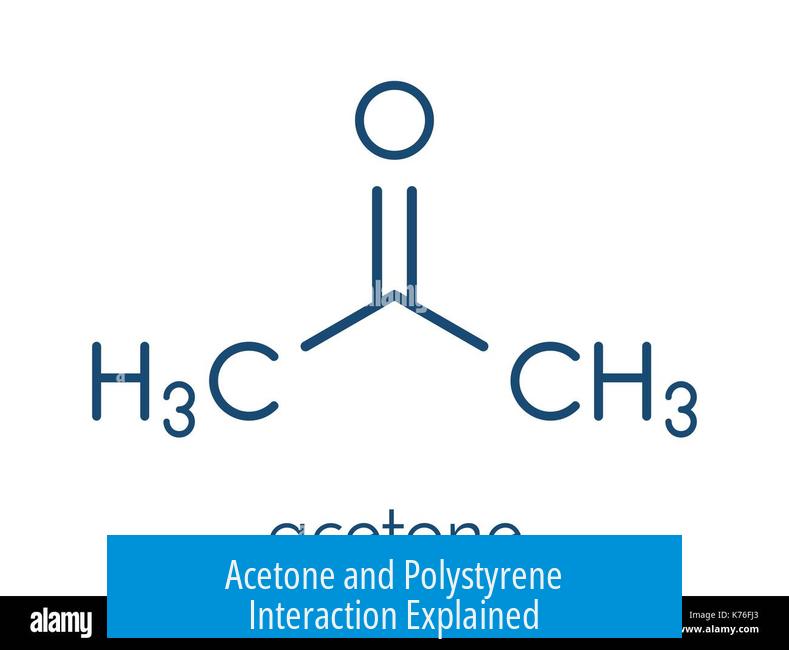
When acetone interacts with polystyrene foam, it causes the polystyrene to physically expand by soaking up the acetone at a molecular level, contrary to the initial appearance of shrinking. This process is central to understanding the behavior of polystyrene in acetone and explains the foam’s transformation during exposure.
Polystyrene Foam Structure and Initial Behavior

Polystyrene foam consists of tiny solid beads, each containing pentane in their cores. Pentane acts as a blowing agent, expanding the beads to form the foam during manufacturing. Upon contact with acetone, this foam behaves differently than expected.
- Although visually the foam appears to shrink, it is actually expanding internally as it absorbs acetone.
- The visible shrinking occurs because the foam’s bead structure breaks down, disrupting the foam’s volume distribution.
Role of Pentane and Acetone
The pentane trapped within the polystyrene beads plays a crucial role. Acetone dissolves this pentane vapor, contributing to the disintegration of the foam:
- Most pentane remains trapped initially but gets dissolved by acetone over time.
- This dissolution weakens the bead structure, leading to the collapse of the foam matrix.
Gelation and Reversible Expansion
When polystyrene foam becomes saturated with acetone, it forms a semi-solid gel:
- The gel shrinks and hardens as acetone evaporates.
- Upon re-immersion in acetone, the gel softens and expands again.
This cycle results from acetone dissolving into and evaporating out of the polystyrene, showing a dynamic solvent-polymer interaction rather than pure dissolution or suspension formation.
Similar Phenomena in Other Polymers
Similar solvent-induced swelling is observed in rubber exposed to chlorinated solvents like dichloromethane:
- The rubber absorbs the solvent and swells substantially without dissolving.
- The volume increase is caused by solvent molecules entering the rubber network.
Clarifying the Interaction Nature

Initial thoughts suggested acetone might suspend polystyrene particles without dissolving them. However, the solvent actually dissolves into the polystyrene matrix, causing swelling. This distinction is important for interpreting changes in foam properties.
Key Takeaways
- Polystyrene foam absorbs acetone, expanding internally despite appearing to shrink.
- Pentane vapor inside foam beads dissolves in acetone, aiding foam breakdown.
- Acetone saturation creates a semi-solid gel that hardens as acetone evaporates and re-expands on re-immersion.
- Similar swelling occurs in rubber with chlorinated solvents, caused by solvent uptake rather than dissolution.
- The interaction is best described as acetone dissolving into polystyrene, not merely suspending it.
Acetone and Polystyrene: The Curious Case of Shrinking Foam That Grows
When polystyrene foam meets acetone, it doesn’t behave like you might expect. It appears to shrink, but actually, the material is expanding at the molecular level. This strange reaction has intrigued chemists, hobbyists, and curious minds alike. Let’s dive into the science behind this paradoxical behavior and uncover what’s really going on inside the foam.
Why Does Polystyrene Foam Look Like It Shrinks, But Actually Expands?
When you dunk polystyrene, say a styrofoam cup or packing peanut, into acetone, you’ll notice the foam quickly collapses and looks like it’s disintegrating. But this is a visual illusion. In reality, on a microscopic level, the polystyrene is acting like a sponge.
- The tiny beads of polystyrene soak up the acetone, causing the material to expand overall.
- What you see is the foam balls disrupting, causing the large, airy structure to lose its shape, which looks like shrinking.
This is because the polystyrene beads absorb acetone between their molecules, causing the solid polymer to swell. The foam structure breaks apart, but the polymer itself takes up more space as acetone dissolves into it.
The Secret Inside the Foam: Pentane and Beads
Ever wondered why polystyrene foam feels so light and puffy? It’s thanks to pentane—a hydrocarbon trapped inside tiny solid polystyrene beads. These beads expand into foam when heated, with pentane acting as the blowing agent.
- Each bead is like a tiny balloon filled with pentane vapor.
- When you apply heat, pentane vaporizes, inflating the beads into foam.
When you later expose this foam to acetone, things get interesting. The pentane vapor trapped inside starts dissolving into the acetone. This helps the foam break down because the “balloons” lose their internal gas, destabilizing the foam’s structure.
From Solid Foam to Semi-Solid Blob: The Role of Acetone
If you let the foam soak long enough, the polystyrene beads swell so much that they merge into a semi-solid, gel-like blob. This blob is saturated with acetone, which acts as a solvent.
- As acetone evaporates, the blob shrinks and hardens.
- Put the hardened blob back into acetone, and it softens and expands again.
Think of this as a reversible dance between polystyrene and acetone. The acetone dissolves into the polystyrene, swelling it. When acetone evaporates, it shrinks and stiffens, only to soften again when re-exposed to the solvent.
Is It Dissolving or Just a Suspension?
At first glance, it might seem like acetone just suspends bits of polystyrene without truly dissolving it. But closer inspection reveals acetone actually dissolves into the polymer chains.
So, the polystyrene doesn’t merely float around as suspended chunks; acetone molecules penetrate the polymer matrix, gently loosening its structure and swelling the material.
How Does This Compare to Other Materials?
The swelling effect isn’t unique to polystyrene and acetone. Similar behaviors are found in other polymers and solvents.
- Rubber and chlorinated solvents: Drop a rubber object into solvents like dichloromethane (a chlorinated solvent), and it will swell dramatically, sometimes multiple times its original size.
- The solvent dissolves into the rubber matrix, causing it to expand without dissolving completely.
This parallel helps to clarify the process: solvent molecules enter the polymer, infiltrating and expanding it, but without fully dissolving into a liquid state immediately.
What Can We Learn From This?
This interaction helps explain why acetone is such a good polystyrene dissolver—and why this “dissolution” behaves differently from what you’d expect with many solids.
If you’re thinking about using acetone to get rid of pesky styrofoam packing peanuts clogging your workspace, here’s what to expect:
- Pour acetone over polystyrene foam.
- Watch the structural integrity fade as pentane vapor dissolves and escapes.
- Notice the foam collapse without it actually shrinking in polymer volume.
- Let the mixture dry and solidify as acetone evaporates, forming a hard, shrunken blob.
- Dip that blob back into acetone to make it expand and soften again, almost like magic.
Practical Tips and Warnings
If you want to experiment:
- Use a well-ventilated space—acetone is volatile and flammable.
- Avoid skin contact; acetone dries out skin and can irritate.
- Don’t pour dissolved polystyrene down drains; it can clog pipes.
- Dispose of acetone-polystyrene waste responsibly.
Remember, while acetone dissolves the foam, it’s not a quick vanish—it’s a process. The foamy mess reduces dramatically, but the polymer remains; it just changes form.
Final Thoughts: Beyond Fun Science
Polystyrene and acetone provide a stunning, tangible example of polymer-solvent interactions. This knowledge reaches far beyond party tricks with packing peanuts.
Industries use solvent interactions like these routinely in recycling, manufacturing, and chemical processing. Understanding molecular expansion and contraction helps innovate better materials and more efficient recycling methods.
So next time you watch polystyrene foam collapse under acetone, you’re seeing molecular drama: a sponge-like swell, the escape of trapped vapors, and the surprising reversibility of polymer swelling.
Isn’t chemistry fun when it bends reality so entertainingly?
What happens to polystyrene foam when exposed to acetone?
Polystyrene foam soaks up acetone like a sponge and physically expands. Although it looks like it shrinks, the foam’s tiny beads swell as acetone dissolves inside them.
Why does polystyrene foam appear to shrink in acetone even though it expands?
The foam’s small solid beads disrupt and pack closer together when acetone dissolves trapped pentane vapors. This disruption creates an illusion of shrinkage despite actual expansion inside.
How does acetone affect the pentane inside polystyrene foam?
Acetone dissolves the pentane vapor trapped in the foam’s beads. This dissolution helps break down the foam structure and leads to foam disintegration.
What happens to semi-solid polystyrene saturated with acetone when acetone evaporates?
The gelled polystyrene shrinks and hardens as acetone leaves. When soaked again, it softens and expands due to acetone dissolving back into the material.
Is the polystyrene dissolving in acetone or forming a suspension?
Acetone does not just suspend polystyrene but actually dissolves into it. This causes swelling and softening, confirming true dissolution rather than a simple suspension.
How does this interaction compare to rubber in chlorinated solvents?
Rubber swells in chlorinated solvents like dichloromethane as the solvent dissolves into it. Similar to polystyrene with acetone, rubber expands without dissolving completely.





Leave a Comment