Is Kinetic Energy Equal to Heat?
Kinetic energy is not equal to heat. Heat is a mode of energy transfer, while kinetic energy is the energy of motion. They describe different physical concepts and cannot be used interchangeably.
Difference Between Kinetic Energy and Heat
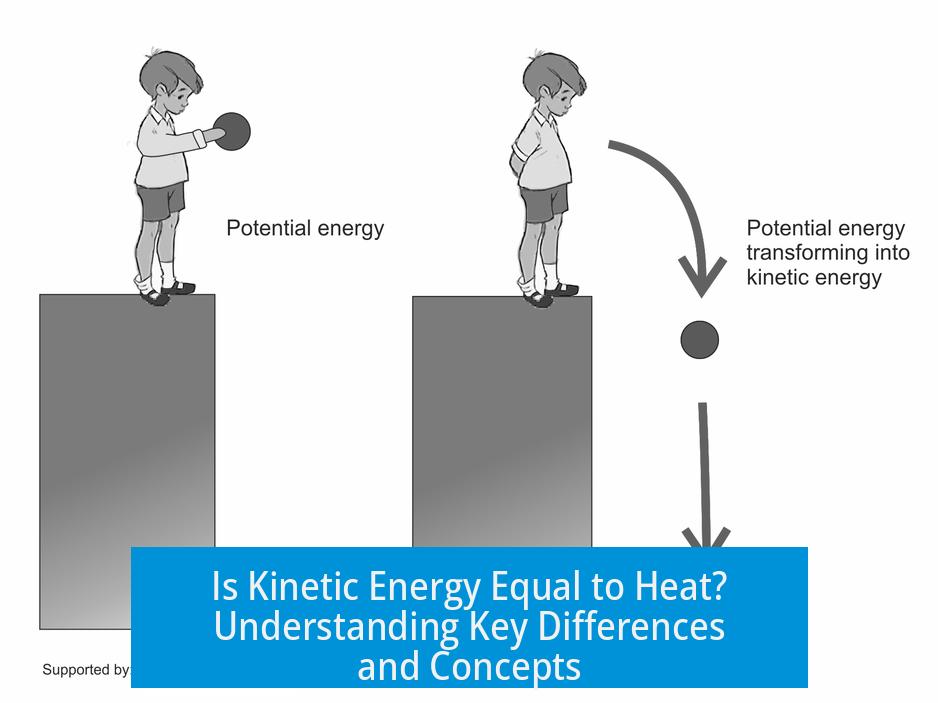
Kinetic energy measures the energy resulting from an object’s motion. This includes the directed motion of an entire body, such as a rolling ball or a speeding asteroid. For example, the interstellar asteroid Oumuamua moves at high speed and has large kinetic energy. Yet, it remains cold in space because temperature relates to particle movement, not bulk motion.
Heat, on the other hand, is the process of energy transfer due to a temperature difference. Unlike kinetic energy, heat is not something stored inside an object. A hot stove does not contain “heat” as a substance; rather, it possesses a higher temperature and can transfer energy as heat to other bodies in contact.
Understanding Temperature vs Heat
Temperature quantifies the average random kinetic energy of particles in a substance. This random motion causes particles to collide and transfer energy microscopically. Heat occurs when this energy moves from a hotter object to a cooler one.
Heat is an energy transfer mode, not a form of energy itself. It describes how energy is redistributed between systems, unlike kinetic energy which is a physical energy stored due to motion. This distinction is key in thermodynamics.
Analogies Clarify the Concepts
Imagine a crowd of people. If they all walk in the same direction at the same speed, this is similar to bulk kinetic energy—organized motion. If the crowd moves erratically in different directions, it resembles random kinetic energy contributing to temperature.
Heat transfer corresponds to energy passing between these groups due to differences in their random motion, not the overall directed motion.
Summary of Key Points
- Kinetic energy is the energy due to motion of an object as a whole.
- Heat is the transfer of energy caused by temperature differences.
- Temperature relates to random kinetic energy of atoms or molecules.
- Heat is not stored energy; it is a mode of energy transfer.
- Bulk kinetic energy (organized motion) does not determine temperature or heat directly.
Is kinetic energy the same as heat?
No, kinetic energy and heat are not the same. Kinetic energy is the energy of motion, while heat is energy transferred between bodies due to temperature differences.
Can an object have high kinetic energy but be cold?
Yes. For example, a fast-moving asteroid has high kinetic energy but can still be cold because temperature depends on the random motion of particles, not bulk motion.
How does temperature relate to kinetic energy?
Temperature measures the random kinetic energy of a substance’s particles. It reflects particle motion that causes collisions, not the object’s overall movement.
Is heat a form of stored energy?
Heat is not stored energy. It is energy in transfer between systems due to temperature differences, similar to work, which also transfers energy.
What analogy helps explain kinetic energy versus heat?
A parade of people walking in the same direction represents bulk kinetic energy. A panicked crowd moving randomly, colliding, and changing speed represents heat as random particle motion.



Leave a Comment