Is There Any Version of the Ideal Gas Law Where the Units Are Defined So That R=1?

There is no standard or commonly accepted unit system in which the gas constant \( R \) is exactly equal to 1. However, certain unit conventions and natural unit systems allow simplifying or absorbing \( R \) or related constants, but not to the extent of having \( R=1 \) directly as a universal constant in the ideal gas law.
Understanding the Gas Constant \(R\) and Its Units
The ideal gas law appears most commonly as PV = nRT, where:
- P = pressure
- V = volume
- n = number of moles
- R = gas constant
- T = temperature (Kelvin)
The gas constant \( R \) is a proportionality factor that balances the units on both sides of the equation. It fundamentally relates the microscopic and macroscopic properties of gases through the universal gas constant.
Importantly, the exact numerical value of \( R \) depends on the units chosen for pressure and volume. The number of moles \( n \) and temperature \( T \) are generally fixed in mole and Kelvin, respectively.
For example, when pressure is measured in atmospheres (atm), volume in liters (L), and temperature in kelvin (K), \( R \) has the value:
| Units | Value of \( R \) |
|---|---|
| L·atm/mol·K | 0.0821 |
Alternatively, if pressure is in pascals (Pa) and volume in cubic meters (m3), \( R \) takes the value:
| Units | Value of \( R \) |
|---|---|
| J/(mol·K) | 8.314 |
The Dependence on Units
\( R \) is not a pure number because its dimensions compensate for the units on pressure, volume, moles, and temperature. As a result, changing pressure or volume units alters the magnitude of \( R \). However, the constant cannot be unitless unless the units themselves are specially designed or adjusted.
Unit Systems and Attempts to Normalize \(R=1\)
Natural and Planck Units

Physicists often work with systems where fundamental constants become unity to simplify equations. For the ideal gas law, it is possible to choose units where the Boltzmann constant \( k_B = 1 \) rather than \( R \).
The Boltzmann constant relates the gas constant \( R \) to Avogadro’s number \( N_A \):
\( R = k_B \times N_A \)
By fixing \( k_B = 1 \), temperature is measured in units of energy. Then, the ideal gas law can be rewritten as:
\( pV = N T \)
Where:
- \( p \) = pressure
- \( V \) = volume
- \( N \) = total number of particles (molecules)
- \( T \) = temperature in energy units
In this form, \( R \) no longer explicitly appears; it is absorbed into the definitions of the quantities. However, this does not imply \( R=1 \) but rather that the concept of \( R \) is replaced by other constants set to unity.
Hypothetical Unit Redefinitions
One could invent a new absolute temperature unit (for example, “Jazzy”) defined such that:
1 Jazzy = \( \frac{1}{R} \) kelvin
In this invented system, the ideal gas law would become:
\( PV = n \times 1 \times T_{Jazzy} \)
This implies \( R = 1 \) in the new units. However, such units are theoretical and nonstandard, devised simply to simplify the equation. The practical usefulness of this approach is limited, and no scientific fields have widely adopted it.
The Practical View: Why \( R = 1 \) Is Uncommon
Chemists and engineers stick to established unit systems and standard constant values, like:
- \( R = 8.314 \, J/(mol \cdot K) \)
- \( R = 0.0821 \, L\cdot atm/(mol \cdot K) \)
These values are ingrained in literature, calculations, experiments, and teaching. Redefining \( R = 1 \) would require wholesale changes in unit conventions and calibration, with little added benefit.
Moreover, \( R \) connects macroscopic measurements with microscopic physics. Changing it to unity would blur the distinction between physical properties and unit systems.
Physicists Versus Chemists
Physicists prefer unit systems where dimensionful constants are unity for simplifying theoretical models (like Planck units or natural units). Chemists generally use practical macroscopic units grounded in experiment and industry standards.
In physics, setting \( k_B = 1 \) simplifies statistical mechanics and thermodynamics. In chemistry, conventional \( R \) values simplify stoichiometric and thermodynamic computations without unit ambiguities.
Variations of \(R\) in Different Units
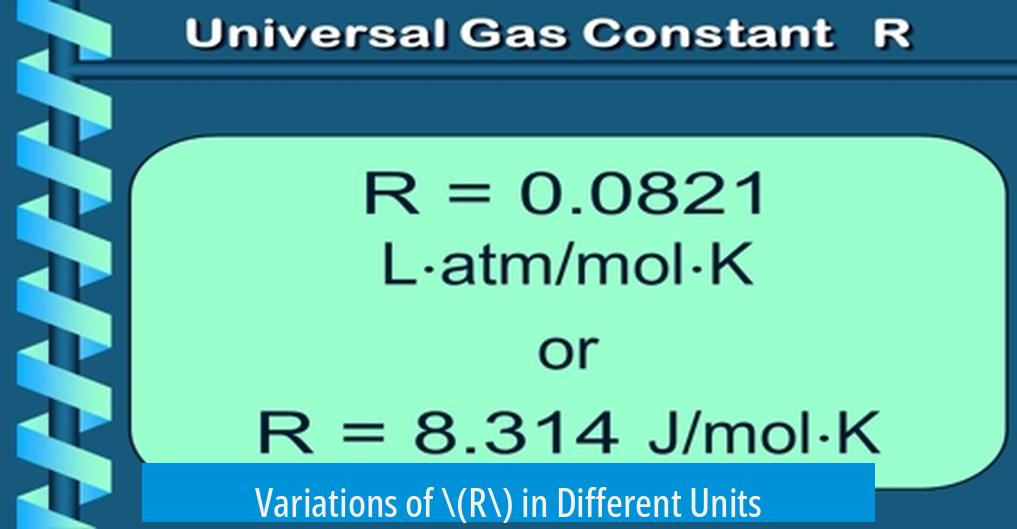
The value of \( R \) changes depending on the units applied. For example:
| Unit System | Value of \( R \) | Units |
|---|---|---|
| SI Units | 8.314 | J/(mol·K) |
| Atmosphere-Liter | 0.0821 | L·atm/(mol·K) |
| British Thermal Units (Btu) | ~1.987 | Btu/(lb-mol·°R) |
The range shows the flexibility but also highlights that \( R \) seldom aligns with an integer or unity. Its value hovers around 1 or 2 in certain systems, but never exactly 1 with standard units.
Summary of Key Points
- No known, standard unit system fixes \( R = 1 \) exactly for the ideal gas law.
- Physicists commonly set the Boltzmann constant \( k_B = 1 \), which effectively absorbs \( R \) into fundamental units.
- Inventing new units (e.g., temperature units scaled by \( 1/R \)) can normalize \( R=1 \), but such units are non-standard.
- Chemists and engineers use conventional values of \( R \) tied to well-defined units and measurements.
- Changing \( R \) to unity would disrupt established conventions without practical gains.
Is There a Version of the Ideal Gas Law Where R = 1?
Short answer: No standard or commonly accepted version of the Ideal Gas Law has the gas constant R equal to exactly 1. But wait—there’s some interesting nuance here involving physics, units, and playful unit inventions!
The gas constant R is a bit like the Swiss Army knife of thermodynamics: its value depends heavily on the units chosen. So, let’s unravel this fascinating topic together with a sprinkle of humor and clarity.
The Chameleon Nature of the Gas Constant (R) and Its Units
The Ideal Gas Law, PV = nRT, where:
- P = pressure
- V = volume
- n = number of moles
- R = gas constant
- T = temperature (in Kelvin)
The constant R changes its numerical value with the choice of units for pressure and volume—but moles and Kelvin are generally fixed. For example, when using atmospheres for pressure and liters for volume, students often see R as 0.0821 L·atm / mol·K. Change your units, and the number shifts.
In fact, use British thermal units (Btu), pounds-moles, and degrees Rankine, and R hovers near 2. This shows that the number itself doesn’t have some magical universal fixed value; it’s a product of how humans tweaked their measurement systems.
But What About R = 1? Why Not Just Define Units That Way?
A clever idea is to invent units where R equals exactly 1. For example, you could define a new temperature scale—the Jazzy—that equals 1/R in Kelvins.
Imagine reporting temperatures in Jazzies instead of Kelvins. It’s playful, a little wild, but possible. You’d rewrite the Ideal Gas Law without an awkward constant term:
PV = n × 1 × TJazzy
However, this is purely hypothetical—there’s no such official scale, and chemists aren’t lining up to switch. After all, this would make their unit conversions a mess, textbook tables obsolete, and lab reports confusing.
Physicists and the Magic of Natural Units: Setting Boltzmann’s Constant kB = 1
Now here’s where the story gets fascinating. Physicists often sidestep the whole R debate by working in natural units, especially Planck units, where they set the Boltzmann constant kB equal to 1.
Why? Because R = NA × kB (Avogadro’s number times Boltzmann’s constant). So, if kB = 1, then R essentially becomes Avogadro’s number, and the ideal gas law simplifies dramatically.
In these units, temperature naturally takes on the units of energy, not something stranger like “Jazzy.” The Ideal Gas Law reduces to the elegant:
pV = N T
Here, N is the number of particles, and T is measured in energy units, absorbing what R would normally represent.
That’s a physicist’s way of cleansing the kitchen counter before cooking: they tidy units so constants vanish, letting the core physics shine.
So Why Don’t Chemists Do This? Practical Concerns and Tradition
Chemists usually stick with traditional units (R ≈ 8.314 J/mol·K, or 0.0821 in liter·atm/mol·K). Why? Because the value R is etched in their brains, textbooks, and lab instruments. Using a different unit system that sets R = 1 adds needless complexity to practical calculations.
Think about converting centuries of measurements, logs, and empirical data into a new fanciful unit. Not a small feat. And really, does setting R to 1 help them in real-world lab work? The consensus seems to be “no.”
How Does This All Impact Learning and Research?
For students and researchers, understanding that R is not a fixed number but depends on units helps avoid confusion. It also offers a cool peek into how different scientific fields juggle constants and units differently.
Physics plays with natural units to make equations slick and reduce clutter. Chemistry prefers fixed, reliable constants for day-to-day accuracy. Engineering lies somewhere in between.
If you’re eager to tinker, imagine defining your own temperature scale or volume unit to normalize R. It’s an intellectual game, but remember that practical experiments, conversions, and communication need stability in units.
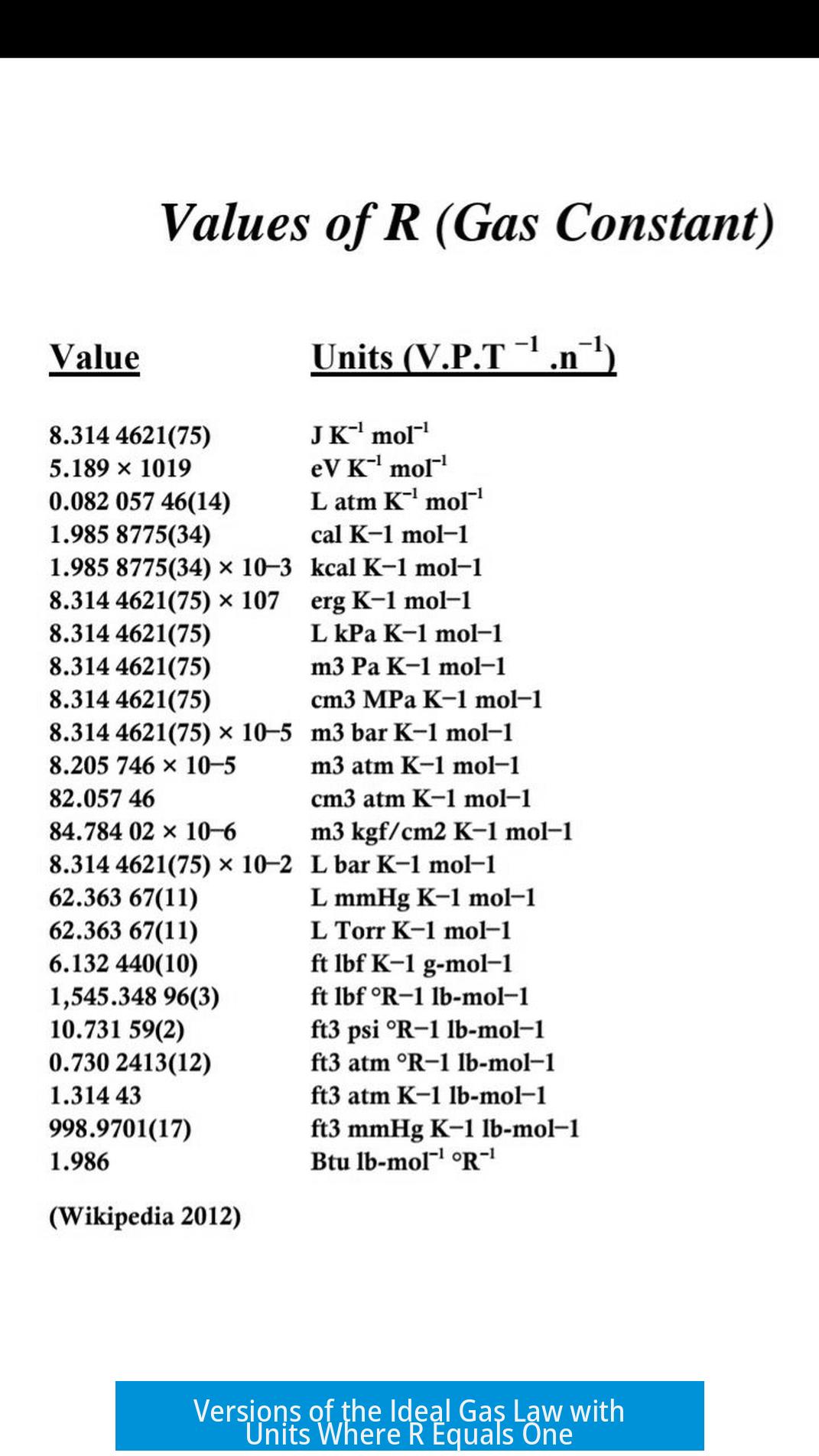
Summary Table: Gas Constant Values in Different Units
| Unit System | Value of R | Comments |
|---|---|---|
| Liter, Atmosphere, Mole, Kelvin | 0.0821 L·atm/mol·K | Common in chemistry labs |
| Joule, Mole, Kelvin | 8.314 J/mol·K | SI standard |
| Btu, lb-mol, Degree Rankine | About 2 | British engineering units |
| Planck Units (Natural units) | Not defined; kB=1 | Physics simplification, R absorbed |
Final Thoughts
Is there a version of the Ideal Gas Law with R = 1? Not in any standard system. It’s a beautiful thought experiment to invent units like the Jazzy, but not practical in chemistry or engineering.
If you ever stumble upon a physicist bragging that kB = 1, now you know this is their sneaky way of simplifying PV = nRT to pV = NT, trading a pesky constant for a particle count and an energy-like temperature.
In the end, R is much more about context and units than a single absolute value. So next time you see that 8.314 or 0.0821, smile—those numbers are playful, unit-driven characters in the grand theatre of physics and chemistry.
Curious for more? Wikipedia hosts a treasure chest of unit systems and R values for various contexts—explore the wonderful world of gas constants and see how far human ingenuity in unit crafting can really go!
Can the Ideal Gas Law be expressed with units where the gas constant \(R = 1\)?
No standard system or units exist where \(R\) is exactly 1. The gas constant depends on chosen pressure and volume units, and common versions use \(R \approx 0.0821\) or 8.314 with standard units.
How do physicists simplify the Ideal Gas Law without using \(R\)?
Physicists often set Boltzmann’s constant \(k_B = 1\). This makes temperature measured in energy units and rewrites the law as \(pV = NT\), avoiding \(R\) entirely.
Is it possible to invent units so that \(R = 1\) in the Ideal Gas Law?
One could hypothetically create a new temperature unit, like the Jazzy, where 1 Jazzy = \(1/R\) kelvin. This would make \(R=1\), but it’s not a standard or practical unit system.
Why don’t chemists use units that make \(R = 1\)?
Chemists rely on well-established values of \(R\), such as 8.314 J/mol·K. Changing units to make \(R=1\) offers little practical advantage and is typically not done.
How does the value of \(R\) change with different unit systems?
The value of \(R\) varies depending on units for pressure and volume. For example, in Btu per pound-mole and degrees Rankine, \(R\) is near 2, showing unit choice affects its numeric value.



Leave a Comment