Understanding the Inverse Dean Stark Condenser Setup

An inverse Dean Stark condenser reverses the traditional flow of liquid and vapor to enable efficient continuous drying or solvent removal during distillation processes. This variation adapts the classic Dean Stark apparatus, improving its application in certain chemical procedures.
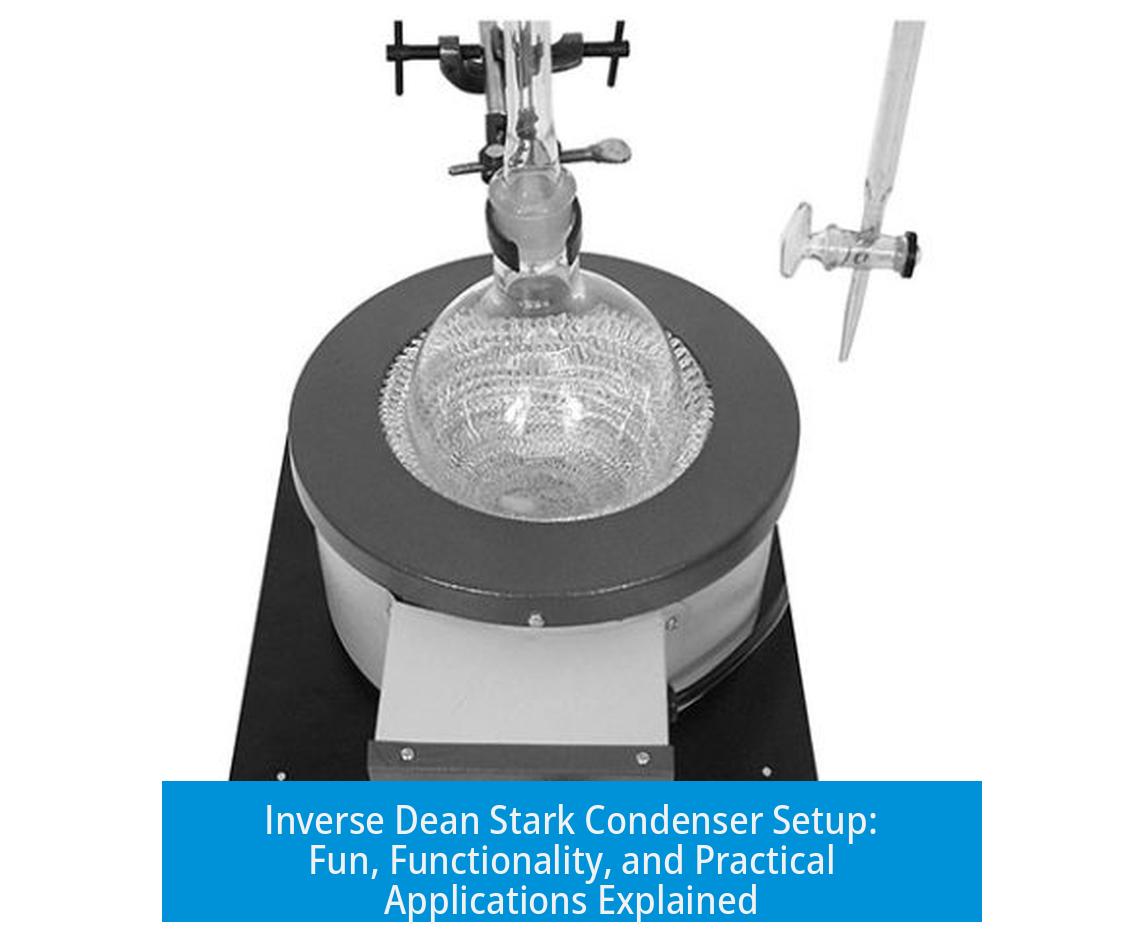
Description and Functionality
The inverse Dean Stark condenser modifies the conventional setup by reversing the direction of reflux and collection. Instead of the typical downward condensate flow, it channels vapor and liquid phases opposite to the usual arrangement. This unique approach allows integrating drying agents, such as molecular sieves, directly within the reflux path. As the solvent vapor condensates, it passes through sieves that absorb water or other impurities, keeping the solvent dry throughout the process.
Purpose and Practical Applications

- Continuous solvent drying during reflux distillations.
- Removal of low-boiling contaminants like methanol by trapping water or other unwanted components.
- Continuous extraction or reagent purification where steady separation is necessary.
By incorporating sieves in the reflux loop, the setup ensures the solvent remains anhydrous while distilling. This is particularly useful in reactions sensitive to moisture or in solvent recovery steps. The setup shares similarities with continuous extraction systems, which maintain steady separation and purification over time.
Comparisons and Alternatives
While a Soxhlet extractor offers continuous solvent extraction by cycling solvent through a solid matrix, the inverse Dean Stark setup primarily focuses on continuous co-distillation with in-line drying. Soxhlet is efficient for solid-liquid extractions but may not provide solvent drying simultaneously. Conversely, this inverse design supports both continuous solvent drying and component separation.
This versatile apparatus can also be experimented with for continuously washing an organic phase of lower density using water or other solvents. Its continuous reflux and separation potential provide flexibility beyond classical Dean Stark or Soxhlet systems.
Related Apparatus and Observations

The inverse Dean Stark exhibits some design features also found in the Clevenger apparatus, used for essential oil extraction via steam distillation. Both employ continuous phase separation and reflux but target different compounds and processes.
Such setups often draw admiration for their clever design and show experimenters’ creativity in optimizing complex distillations.
Key Takeaways
- The inverse Dean Stark condenser reverses flow to enable continuous solvent drying with integrated sieves.
- It suits processes needing steady removal of water or volatile impurities during reflux.
- Offers advantages over Soxhlet extractors by combining drying and distillation functions.
- May adapt to continuous washing or extraction involving phase separations.
- Shares operational concepts with the Clevenger apparatus used in steam distillation.
Exploring the Quirky World of an Inverse Dean Stark Condenser Setup

Ever heard of an inverse Dean Stark condenser? It’s a clever twist on a classic chemistry tool, designed to make solvent removal and drying a bit more efficient—and yes, much more fun to use. The traditional Dean Stark apparatus is straightforward: it traps and measures water or other condensable liquids during reflux and distillation. But when you flip the idea on its head, creating an “inverse” version, things get interesting.
So, what’s happening here? The inverse Dean Stark condenser changes how the reflux and condensation flow within the apparatus. Picture reversing the usual path of vapors and liquids to suit a special purpose. This setup can be tailored to specific lab work, such as drying solvents directly during reflux, which saves time and hands-on fiddling.
What Makes the Inverse Dean Stark Setup Tick?
The “fun set up” part comes with integrating sieves inside the reflux path. These aren’t just decoration—silica or molecular sieves play a crucial role by removing traces of water or methanol while the solvent boils. Imagine the solvent refluxing nonstop, and as it hits the sieves, it’s continuously dried before cycling back. This arrangement intensifies the drying process without needing to stop and swap out materials repeatedly. It’s like having a built-in solvent spa treatment.
Why is drying solvent so important? Many reactions or extractions demand water-free solvents. Water can ruin sensitive syntheses or interfere with extraction efficiency. Traditionally, chemists might add drying agents separately or distil solvent repeatedly. This setup makes the process “hands-off” and continuous.
Does It Handle Methanol Removal Well?

Some users wonder if this setup tackles methanol removal, especially given methanol’s low boiling point and tricky behavior in mixtures. The answer lies in the sieves themselves. Depending on the choice, they can absorb methanol or water residuals selectively. So yes, if your process produces methanol that you need to get rid of, the sieves inside the inverse Dean Stark might just be your best friends. They work while letting the rest of your solvent recycle smoothly.
In essence, you have a system that both dries and purifies, which can be critical in complex chemical or natural product extractions.
Is This Like a Continuous Extraction Setup?
The answer is a bit “yes and no.” Traditional continuous extraction often involves apparatuses like Soxhlet extractors, which cycle solvent repeatedly over solid material to extract components. The inverse Dean Stark can feed into continuous processes by maintaining solvent purity and dryness, but its main role remains solvent separation and drying during reflux. So, we could call it a “continuous solvent maintenance setup” rather than a pure extractor.
How Does It Compare to a Soxhlet?

The Soxhlet extractor lives for relentless extraction—flowing solvent over solid samples with repetitive washing cycles. However, it doesn’t inherently dry the solvent inside the apparatus. That’s where the inverse Dean Stark earns bonus points. It can dry the solvent mid-process, preventing water buildup and improving extraction consistency.
For those wondering why not just use a Soxhlet: the inverse Dean Stark setup is unique because it’s dual-purpose. You get dehydration and solvent removal in one vessel. Soxhlet is fantastic but doesn’t handle solvent drying mid-cycle unless you add external bathes or dry materials separately.
Can You Push This Setup Further? Continuous Washing, Anyone?
Great question! Could this reverse flow system help you wash a lower-density organic layer continuously with water, for instance? Absolutely, with tweaking. The apparatus’s design lets phases separate as they condense, so in theory, you can continuously refresh and wash organic solvents within the same cycle.
This could be a game changer for chemical processes needing repetitive organic-aqueous phase interactions without manual intervention. Think of the time saved and minimized exposure to harmful solvents.
Labels: Mad Science or Practical Innovation?
A lighthearted observer called this rig a “truly mad scientist setup,” complete with sparks from a Tesla coil. Well, it’s quirky, but also smart. It blends creative thinking with serious chemistry efficiency.
Another pointed out similarities to a Clevenger apparatus, widely used to isolate essential oils. That’s correct! Both setups focus on phase separation during distillation, though the inverse Dean Stark is more specialized for drying and solvent purification. This resemblance helps highlight the versatility of reflux-based condensers when tweaked wisely.
What’s the Bottom Line?
Whether you’re a lab veteran or a chemistry hobbyist, an inverse Dean Stark condenser system brings innovation to a classic procedure. It compresses multiple steps—drying, separation, and solvent recycling—into one elegant reflux dance. It saves time, enhances purity, and keeps your solvent system dry without constant monitoring.
Plus, setting it up and watching those sieves soak up impurities adds a bit of that mad-scientist flair we secretly love. Who said chemistry equipment had to be dull?
Practical Tips for Trying Your Own Inverse Dean Stark Setup:
- Choose sieve material carefully—molecular sieves are great for water, and some select materials target methanol.
- Test your solvent interactions—some solvents might vaporize differently in reverse flow, so keep an eye on efficiency.
- Monitor your reflux rate—too fast or too slow can impact drying quality.
- Consider phase densities if you want to try continuous organic/water washing—proper phase separation matters.
By exploring and customizing the inverse Dean Stark condenser, chemists craft tools that are practical and a little bit playful. It’s an elegant reminder that science benefits from a twist—sometimes literally.
What makes the inverse Dean Stark condenser different from a regular Dean Stark apparatus?
The inverse Dean Stark reverses the usual flow or design. This change helps meet specific needs in solvent removal or continuous extraction that the standard setup might not handle efficiently.
How do sieves improve the functionality of this inverse Dean Stark setup?
Sieves placed inside dry the solvent as it refluxes. This continuous drying improves solvent purity and makes the extraction or distillation more efficient.
Can this setup be used for continuous extraction or washing processes?
Yes, it can operate continuously. The design allows for ongoing extraction or even washing of an organic phase with water, adding versatility beyond traditional batch processes.
Why might someone choose this inverse Dean Stark setup over a Soxhlet apparatus?
The inverse Dean Stark allows for drying during distillation and can handle continuous operations. Soxhlet mainly offers repeated solvent washing without integrated drying.
Is there a connection between the inverse Dean Stark and a Clevenger apparatus?
Both involve phase separation during distillation. However, the Clevenger is tailored for essential oil extraction, while the inverse Dean Stark focuses on solvent removal and drying.




Leave a Comment