How Do Atoms Become Ions? Is it All from Making/Breaking Bonds?

Atoms become ions mainly by gaining or losing electrons, but not all ion formation results from making or breaking chemical bonds. Ion formation can happen through diverse processes including electron transfer during chemical reactions, exposure to high energy radiation, or electrical excitation, without the involvement of bond formation or cleavage.
Ion Formation Through Electron Transfer in Chemical Reactions
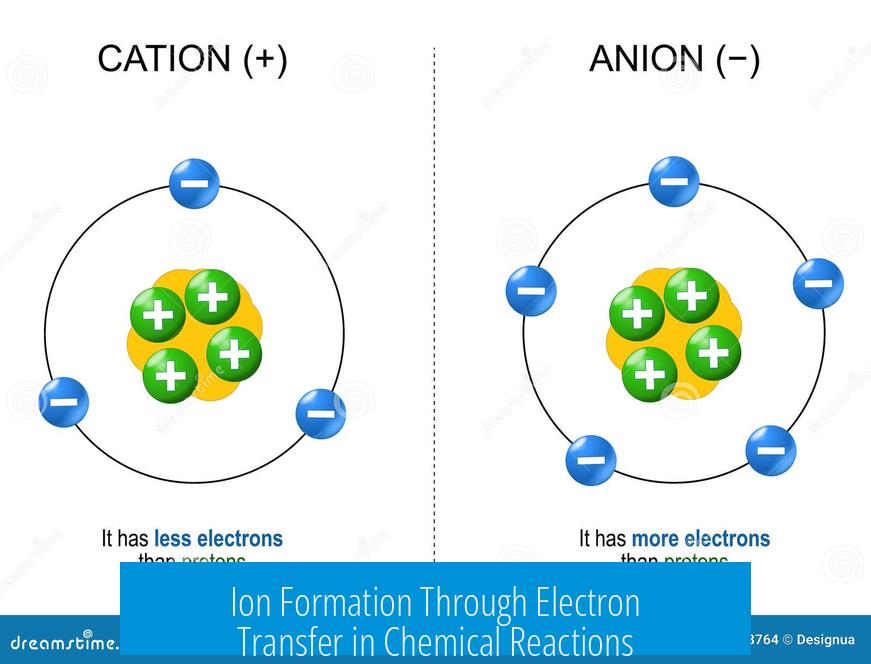
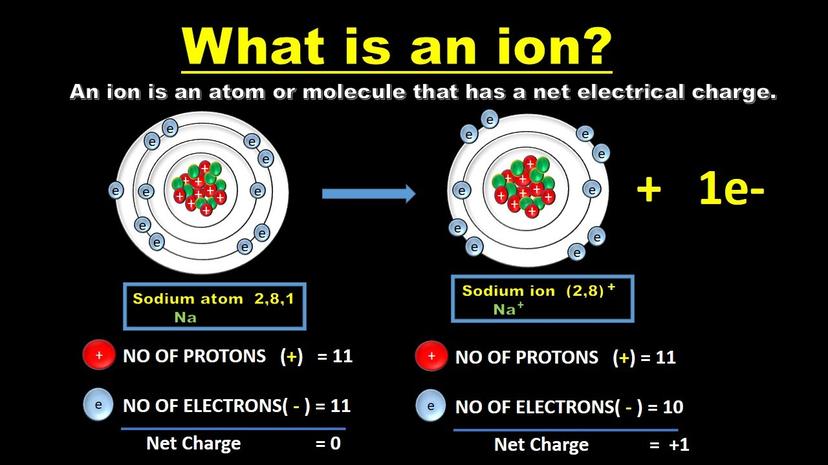
In typical chemical scenarios, ions often form when atoms with differing electronegativities interact. One atom exerts a stronger pull on shared electrons, causing those electrons to transfer fully to this atom. This electron transfer creates positively charged cations and negatively charged anions. For example, sodium reacts with chlorine gas; sodium loses an electron to chlorine, forming Na+ and Cl- ions that bind ionically.
However, many atoms in everyday substances remain neutral. Metals like iron or molecular gases such as chlorine often exist as non-ionized atoms or molecules in their standard state. This shows that ionization is not ubiquitous in chemical bonding.
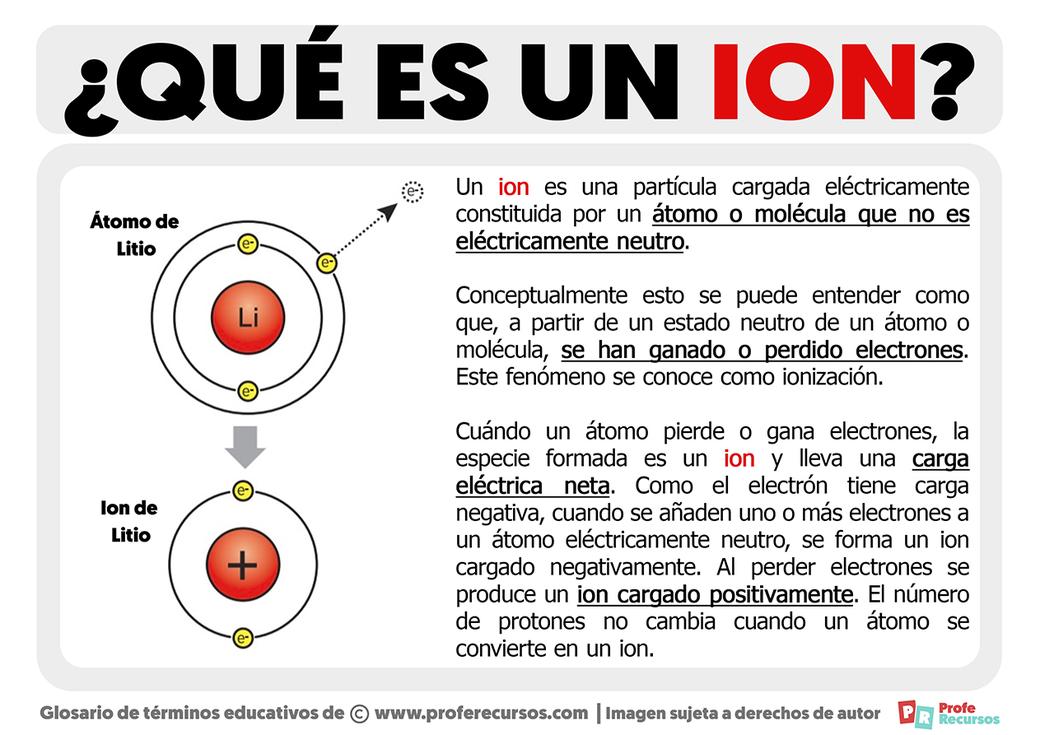
Covalent and Polar Covalent Bonds Do Not Necessarily Produce Ions
When atoms form covalent bonds, they share electrons instead of fully transferring them. If their electronegativities are close, they share electrons equally, resulting in no charge separation. Even in polar covalent bonds, where electrons are shared unequally, electrons are not fully transferred. This partial sharing produces dipoles rather than ions.
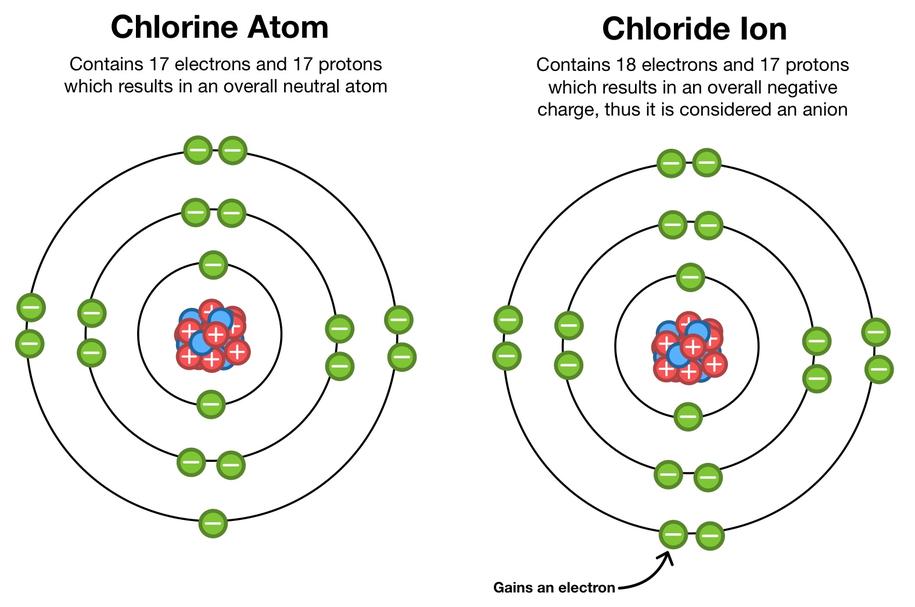
Therefore, making or breaking covalent bonds does not always result in ion formation. Ionization requires complete electron transfer or removal rather than slight electron density shifts within bonds.
Ion Formation Beyond Bond Making and Breaking
Ions can form without any chemical bonding changes. For example, applying electricity or heat can strip electrons from atoms, producing ions. In fluorescent lamps, high voltage removes electrons from gas atoms, generating ionic species required for light emission.
On a cosmic scale, plasma forms when heat or radiation energizes atoms so intensely that electrons detach, creating a soup of ions and free electrons. This state occurs naturally in the sun and lightning and does not depend on chemical bonds.
Summary of Ion Formation Mechanisms
- Ions form by gaining or losing electrons through electron transfer in chemical reactions with large electronegativity differences.
- Many atoms exist as neutrals, and covalent bonds usually involve shared electrons, not ions.
- Polar covalent bonds create dipoles, not ions.
- Electricity, heat, and high-energy radiation can ionize atoms without breaking or making bonds.
- Plasma is a highly ionized state created by extreme energy input, independent of chemical bonding.
How Do Atoms Become Ions? Is It All From Making or Breaking Bonds?
Atoms become ions primarily by gaining or losing electrons, but it is not all about making or breaking chemical bonds. In fact, ions can form through a variety of processes, some involving bonds, and others just high-energy events tearing electrons away. Let’s unpack this mystery with clear facts and examples.
Picture an atom like a cozy house with room for a few guests—electrons. When one atom’s grip on its electrons weakens, or another atom pulls electrons harder, the guest list changes. That’s the heart of how ions are born.
More Than Just Bonds: The Many Paths to Ion Formation
It’s tempting to think that only chemical reactions where bonds break or form create ions. But ions also appear in places with no bond-making or breaking.
- Chemical Reactions: When chlorine gas meets sodium metal, they react chemically. Sodium gives up an electron, becoming Na+; chlorine grabs an electron, becoming Cl−. That’s ions by bond breaking and making.
- Electrochemistry Cells: A battery is a playground for ions. Here, electrical energy prompts atoms to lose or gain electrons, forming ions without classical bond formation.
- High-Energy Events: Ionizing radiation like UV rays, X-rays, gamma rays, and fast neutrons slam into atoms, stripping off electrons and creating ions—no bonds needed.
This range already suggests the simplicity of “making or breaking bonds” doesn’t capture ion formation fully.
Regular Chemistry: Electronegativity’s Tug of War
In everyday chemistry, at room temperature and normal conditions, ions usually appear when atoms have differing strengths in their electron pull, known as electronegativity.
Imagine sodium and chlorine as dance partners: chlorine is a much stronger lead and pulls the electron right off sodium. This electron transfer leaves sodium positively charged (Na+) and chlorine negatively charged (Cl−). The result? Ions.
But does this always happen? Nope. Many atoms hang out as neutral, non-ionized forms, like iron metal, chlorine gas, or paraffin wax. In these cases, atoms either share electrons equally or don’t pull hard enough to strip electrons.
When Bonds Don’t Mean Ions: Covalent Sharing vs Ionic Charge
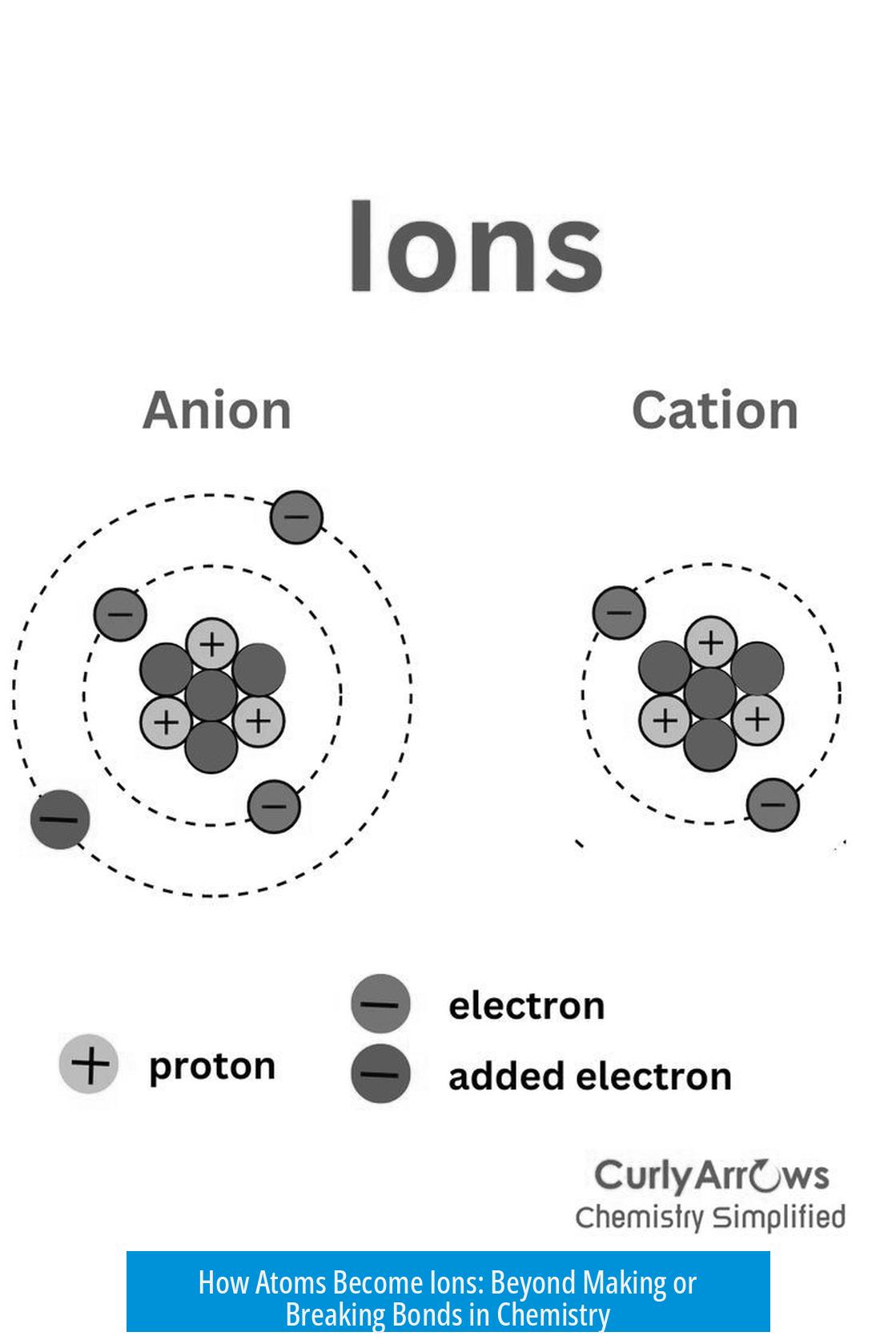
Here’s a fun fact: covalent bonds—where atoms share electrons—usually don’t produce ions. If two atoms have similar electronegativity, they share electrons equally. This creates neutral molecules. Think oxygen in O2 or nitrogen in N2 gas. They’re bonded but not ionic.
Even when sharing isn’t equal, as in polar covalent bonds, atoms don’t become ions. The electrons spend more time closer to the more electronegative atom, creating a dipole but not a full electron transfer. For example, water (H2O) is polar but not made of ions. So polar covalent bonds are a no-go for ion classification.
Ions Can Form Without Making or Breaking Bonds
Let’s venture beyond chemistry class settings. Electricity and heat often create ions without bonds entering the picture.
Take fluorescent lights. Inside, high voltage zaps gas atoms like mercury vapor, tearing away electrons to form positively charged ions. Here, no chemical bonds need to be made or broken—just a pure electrical drive that kicks electrons loose.
Or think about the sun—a giant ball of plasma. Plasma exists when temperatures soar so high that electrons can’t hang on to atoms. These free electrons and ions swirl in a hot soup of charged particles. This is ionization on a cosmic scale, no bond making or breaking required.
Recap: Ion Formation Is a Broader Story
| Method | How Ions Form | Role of Bond Making/Breaking |
|---|---|---|
| Chemical Reactions | Electron transfer due to electronegativity differences | Often involved |
| Electrochemical Processes | Electricity pulls electrons off atoms | Sometimes involved |
| Ionizing Radiation | High-energy radiation strips electrons | Not required |
| Heat/Plasma | Extreme heat frees electrons | Not required |
Why Does This Matter?
Understanding how ions form has real-world benefits. In industry, controlling ion production creates better batteries and lighting. In medicine, ionizing radiation treats cancer by disrupting atomic structures inside cells. Even the tech in your smartphone relies on the behavior of ions in circuits.
Knowing ions don’t just depend on bonds opens doors to new research and tech. Could we someday harness ion formation with sound waves or lasers? Probably. Science always surprises.
A Final Thought
Next time you hear “ions form by making or breaking bonds,” give a little smirk. Atoms have many tricks up their sleeves. Sometimes they share, sometimes they battle over electrons, sometimes they simply lose them to pure energy blasts. Ions remind us that chemistry isn’t just about bonds but about energy, forces, and the invisible tug-of-war electrons play all day long.
So, atoms become ions by gaining or losing electrons through a mix of processes—not exclusively from making or breaking bonds. It’s a fascinating dance involving electronegativity, energy, and sometimes no bonds at all.
How do atoms become ions in typical chemical reactions?
Atoms become ions when one atom pulls electrons from another due to a big difference in electronegativity. The electrons leave one atom and attach to the other, creating ions through electron transfer.
Is ion formation always linked to making or breaking chemical bonds?
No. Ion formation can happen without making or breaking bonds. High voltage or heat can strip electrons directly from atoms, creating ions without changing existing bonds.
Do polar covalent bonds produce ions?
Polar covalent bonds involve unequal sharing of electrons but do not form ions. Electrons stay shared rather than completely moving to one atom.
Can ions form in substances without chemical reactions?
Yes. Ionization can occur from energy sources like UV light or electricity. These sources knock electrons off atoms, forming ions without a chemical reaction.
Are atoms mostly found as ions in everyday materials?
No. Many materials, like iron lumps or chlorine gas, contain atoms that exist primarily in a neutral, non-ionized state.



Leave a Comment