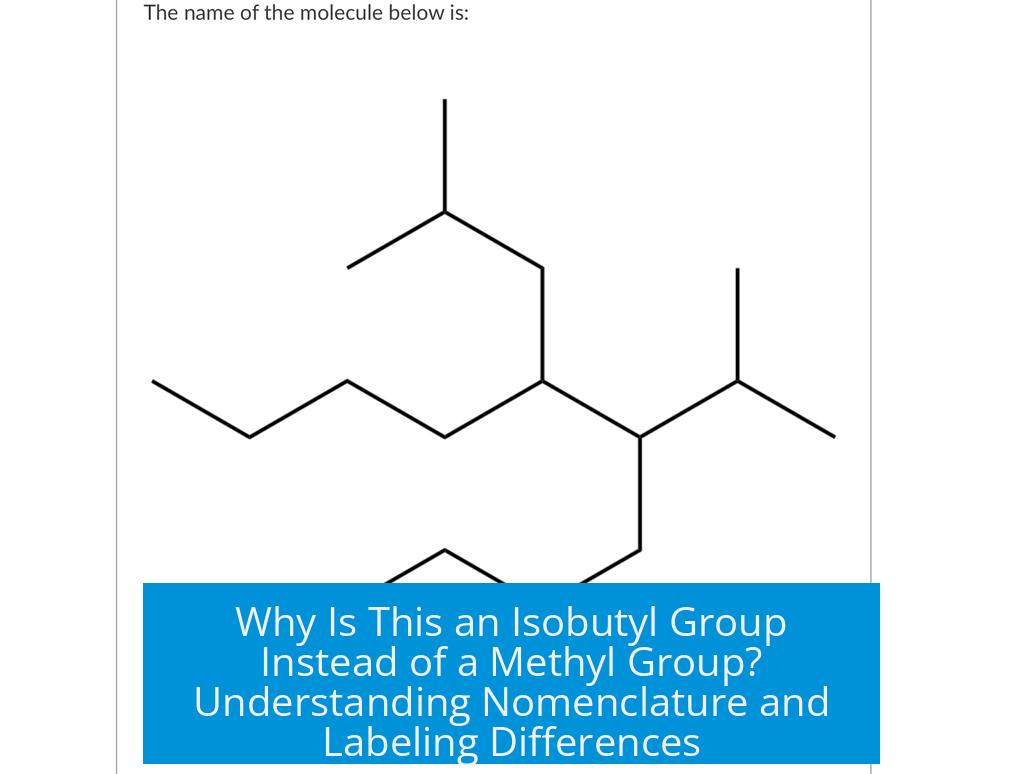
Why Is This Labeled as an Isobutyl Group and Not a Methyl Group?

The group is labeled as an isobutyl group instead of a methyl group because it consists of four carbon atoms arranged with branching, specifically a carbon branch at the second carbon, making it an isobutyl substituent. In contrast, a methyl group contains only a single carbon atom with three hydrogens (–CH3). This distinction is central in common nomenclature where prefixes like “iso-” indicate branching in alkyl groups, unlike the simple, linear methyl group.
Understanding the Difference Between Isobutyl and Methyl Groups
At the core, the difference lies in the number of carbon atoms and how they are connected. A methyl group is the simplest alkyl substituent, containing just one carbon atom bonded to three hydrogens. It forms the basis for many other substituents but remains unbranched and very small.
The isobutyl group, however, consists of four carbon atoms. These form a chain with a methyl branch at the second carbon position. Generalizing from common nomenclature, the prefix “iso-” signals the presence of this branch. Therefore, the isobutyl group is essentially a butyl group (four carbons) with one branch, giving it a L-shaped or branched structure.
- Methyl group: One carbon (–CH3), no branches.
- Isobutyl group: Four carbons total, with a branch at the second carbon.
How Branching Defines Group Labeling
The labeling depends largely on the carbon chain’s branching and length. “Butyl” refers to any four-carbon alkyl group. When one carbon branches off the main chain near the end, the prefix “iso-” is used to specify this structure. The isobutyl name indicates that the carbon chain is not linear but has a branching pattern at the second carbon.
This contrasts with the methyl group labeling, which applies only to a single carbon with no branches. Thus, labeling a segment as methyl is appropriate only if it highlights a solitary –CH3 substituent without additional carbons attached.
Reference Point and Substituent Identity
Where the reference point lies on the molecule influences the group’s label. If only one methyl unit is highlighted, it would be correct to call it a methyl group. However, if the entire four-carbon branched substituent is considered, then the isobutyl label is applied.
The identifying principle in common practice is to emphasize the entire substituent attached to the main carbon chain rather than single constituent methyl groups within that unit. Consequently, the red-highlighted area in the diagram or model often highlights a multi-carbon isobutyl group, not just a methyl part of it.
Common vs. IUPAC Nomenclature: How Labels Differ
Use of Prefixes in Common Nomenclature
Prefixes like “iso-“, “sec-“, “tert-“, and “neo-” are characteristic of common nomenclature. They provide shorthand references for branching and connectivity in alkyl substituents.
- Iso-: A carbon branch at the second carbon from the attachment point.
- Sec-: A substituent attached to a secondary carbon.
- Tert-: A substituent attached to a tertiary carbon.
- Neo-: A substituent with a quaternary carbon center.
The isobutyl group is part of this system. It is a branched alkyl group with the “iso-” prefix marking the pattern of branching. This nomenclature aids in easy communication about molecular structure, especially when discussing alkyl chains informally.
The IUPAC Perspective
IUPAC (International Union of Pure and Applied Chemistry) nomenclature emphasizes systematic naming based on numbering and locating branches precisely. IUPAC rarely uses non-numerical prefixes like “iso-” unless in specific allowed cases. Instead, it uses numbers to locate substituents on the main chain.
From an IUPAC standpoint, the highlighted group might simply be named based on the position and number of carbons attached, which may reduce to a methyl substituent if only one carbon is appended at the correct position. The naming approach avoids ambiguity by focusing on the longest parent chain and exact locations using numbers.
When Do Confusions Arise?
Confusion about labeling arises when diagrams or teaching materials aim to highlight trivial or common names rather than systematic IUPAC names. For example, a group with multiple methyl components may be shown colored, but the overall substituent is larger and branched, requiring an isobutyl designation.
Moreover, poor or inconsistent highlighting and choice of reference chains create ambiguity. If the highlight stops at one methyl but the group conceptually includes more carbons, the name can appear misleading.
Common Pitfalls in Visual Presentation and Labeling
Teaching materials occasionally include examples where color coding or highlights are misleading. A group might be partially highlighted, suggesting a methyl group, while in reality, it is an isobutyl substituent consisting of more carbons.
Such poor execution leads to statements like “the group is methyl technically,” meaning if analyzed strictly, only the highlighted part is methyl. Yet the intent is to demonstrate the difference between butyl group variations using trivial names.
Best Practices for Clarity
- Use consistent and complete highlighting to show the full substituent being named.
- Choose appropriate parent chains for naming to reduce ambiguity.
- Explain when common versus IUPAC nomenclature is being used.
- Clearly label substituent branches to reflect their full structure.
Summary and Key Takeaways
- The isobutyl group includes four carbons with a branching methyl at the second carbon, distinguishing it from a single-carbon methyl group.
- Common nomenclature uses prefixes like “iso-” to describe branching patterns in alkyl groups.
- IUPAC nomenclature focuses on systematic numbering and might label the same group differently, depending on the parent chain and position.
- Visual aids must clearly represent the entire substituent to avoid confusion in nomenclature labeling.
- Branching and total number of carbons determine the appropriate group name, not merely parts of the substituent.
Why is the group labeled as isobutyl instead of methyl?
It is labeled isobutyl because it contains four carbons with a branch on the second carbon. A methyl group only has one carbon (–CH3). The label reflects the entire 4-carbon arrangement, not just a single methyl unit.
What does the prefix “iso-” signify in isobutyl?
“Iso-” indicates a specific branching pattern. In isobutyl, the branch happens at the second carbon, creating a tertiary carbon near the end of the chain. This differentiates it from a straight butyl group.
Could the same group be called methyl in another context?
Yes, if the highlighted part was only one of the methyl groups, it would be called methyl. The name depends on where the reference point is and which part of the molecule is considered the substituent.
Why does common naming use prefixes like iso-, sec-, or tert- instead of strict IUPAC names?
Prefixes like iso-, sec-, and tert- describe branching in common names. IUPAC prefers numerical locants for clarity, so it generally won’t use these prefixes but instead numbers to show the substituent positions.
Is the example in the content showing correct nomenclature?
The example mainly demonstrates common group names and their appearances. It is not strict IUPAC nomenclature. The color coding and chosen parent chain can cause confusion and do not reflect rigorous naming rules.
How can the labeling be clearer in such examples?
Using clearer highlights, extending bonds, or choosing a more appropriate parent chain would help. The example would benefit from showing real substituent positions instead of only trivial group labels.




Leave a Comment