How to Instantly Identify Oxidation and Reduction Reactions in Organic Chemistry
Organic oxidation and reduction (redox) reactions can be instantly identified by observing changes in bonds to oxygen and hydrogen atoms and by analyzing molecular formula changes and reagents. Oxidation generally involves increasing the number of bonds to oxygen or losing hydrogen atoms, while reduction is the opposite. This article explains these indicators in detail to help quickly recognize redox processes in organic chemistry.
Understanding Oxidation and Reduction in Organic Chemistry
In organic chemistry, oxidation and reduction refer to changes in the bonding of molecules rather than simple electron transfers common in inorganic chemistry. The key to identifying these reactions lies in the bonds between carbon and heteroatoms like oxygen and hydrogen.
- Oxidation: A molecule either gains oxygen atoms or loses hydrogen atoms.
- Reduction: A molecule loses oxygen atoms or gains hydrogen atoms.
This simple rule provides a practical shortcut to classify reactions without lengthy analysis.
Bonds to Oxygen and Hydrogen
One of the quickest visual cues is to look at how many bonds to oxygen or hydrogen change during the reaction.
- Increase in bonds to oxygen indicates oxidation. For example, converting a primary alcohol (-CH2OH) to an aldehyde (-CHO) adds a double bond to oxygen, so it is an oxidation.
- Decrease in bonds to oxygen indicates reduction, such as turning a ketone (C=O) into an alcohol (C-OH).
- Increase in bonds to hydrogen also signals reduction—this can be seen when adding hydrogens to a carbon center, saturating double bonds or converting carbonyls into alcohols.
- Decrease in bonds to hydrogen marks oxidation, such as dehydrogenation reactions or formation of double bonds.
Examples of Bond Changes
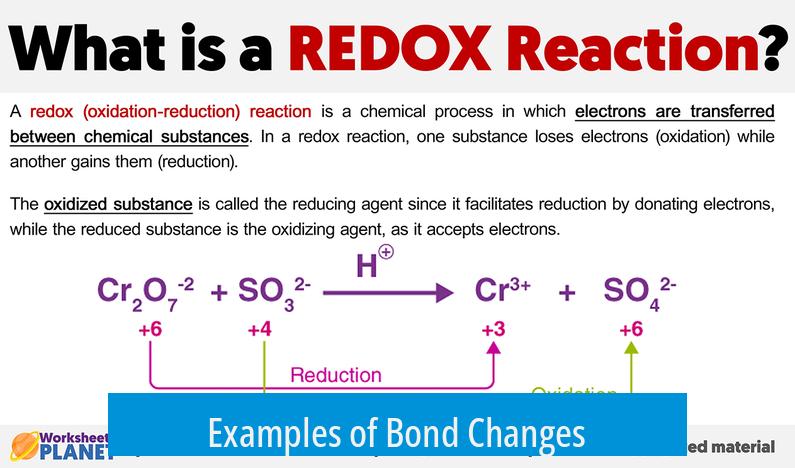
| Reaction | Change | Redox Type |
|---|---|---|
| CH3CH2OH (ethanol) → CH3CHO (acetaldehyde) | Loss of H, gain of C=O | Oxidation |
| CH3CHO → CH3CH2OH | Gain of H, loss of C=O | Reduction |
| CH3CH=CH2 → CH3CH2CH3 | Gain of H (saturation) | Reduction |
Molecular Formula as a Clue
Sometimes structural inspection is difficult, and comparing molecular formulas can quickly indicate redox changes.
- Loss of H2 molecules without any other changes implies oxidation.
- Gain of H2 suggests reduction.
- Gain of oxygen atoms signals oxidation, such as hydroxylation or epoxidation.
- Loss of oxygen atoms points to reduction events.
For example, going from C2H6O (ethanol) to C2H4O (acetaldehyde) involves loss of hydrogen atoms, hence oxidation.
Role of Reagents in Identifying Redox Reactions
Reagents commonly used in organic redox reactions often provide clues about the reaction type.
- Reducing agents usually contain metals with multiple hydrogens, such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4).
- Oxidizing agents are often transition metal compounds rich in oxygen, like potassium permanganate (KMnO4) or chromium-based reagents (CrO42–, Cr2O72–).
When a reaction uses a metal hydride, it likely involves reduction. Oxidations often involve oxidants containing oxygen-rich metal species.
Biochemical Perspective on Redox Identification
From the biochemical standpoint, oxidation typically involves loss of electrons or hydrogen from the molecule, which corresponds with increasing oxygen content or reducing hydrogen content.
For instance, methanol (CH3OH) oxidizes to formaldehyde (CH2=O) by losing hydrogens and gaining a double bond to oxygen. The reverse is a reduction.
This viewpoint aligns well with organic chemistry conventions and offers a consistent method for instant recognition.
Summary Table: Instant Identification of Oxidation and Reduction
| Criterion | Oxidation | Reduction |
|---|---|---|
| Bonds to Oxygen | Increases | Decreases |
| Bonds to Hydrogen | Decreases | Increases |
| Molecular Formula Change | Gains oxygen / loses H2 | Loses oxygen / gains H2 |
| Common Reagents | Transition metal oxidants (KMnO4, Cr2O7) | Metal hydrides (NaBH4, LiAlH4) |
Practical Tips for Quick Identification
- Look at the functional groups: Alcohols converting to aldehydes or ketones signal oxidation.
- Check the number of bonds to oxygen: More C-O or C=O bonds mean oxidation.
- Check for addition or removal of hydrogens on carbons, indicating reduction or oxidation respectively.
- Examine molecular formula changes: Loss/gain of H2 or O atoms points to redox.
- Identify reagents if given. Metal hydrides cause reductions. Metal oxides or permanganates cause oxidations.
Limitations and Considerations
Though these rules are useful, some reactions can be more complicated. Certain rearrangements or radical reactions may not neatly fit these patterns.
Always consider the overall molecular context and electron flow. When uncertain, combine multiple approaches—structural changes, formula changes, and reagents—to confirm the redox nature.
Relevant Resources
- Master Organic Chemistry: Oxidation and Reduction in Organic Chemistry
- Organic Chemistry Teacher Explains Redox on TikTok
Key Takeaways
- Oxidation increases carbon bonds to oxygen or removes hydrogen; reduction does the opposite.
- Molecular formula changes (gain/loss of O or H2) serve as quick redox indicators.
- Reagents identify redox: metal hydrides reduce, oxygen-rich metals oxidize.
- Inspect functional groups and bonding changes for rapid identification.
- Combine approaches for accurate identification in complex cases.
How can I quickly tell if an organic reaction is oxidation or reduction?
Check the bonds to oxygen and hydrogen. If bonds to oxygen increase or bonds to hydrogen decrease, it is oxidation. The reverse, fewer oxygen bonds or more hydrogen bonds, signals reduction.
Can changes in molecular formula indicate oxidation or reduction?
Yes, losing H2 means oxidation, and gaining H2 means reduction. Similarly, gaining oxygen atoms signals oxidation, while losing oxygen indicates reduction.
Do certain reagents instantly reveal if a reaction is oxidation or reduction?
Reagents with metals bonded to hydrogens like NaBH4 or LiAlH4 cause reduction. Transition metal oxides like KMnO4 or Cr2O7 act as oxidizing agents.
Is there a simple rule involving electron changes for these reactions?
Oxidation involves loss of electrons or hydrogens, often with oxygen gain. Reduction involves gain of electrons or hydrogens and usually loss of oxygen.
Does the number of bonds to heteroatoms always determine the redox direction?
Generally, yes. Increasing bonds to oxygen or other heteroatoms means oxidation. Decreasing these bonds means reduction. This is a reliable quick check.




Leave a Comment