Is This a Meso Compound?
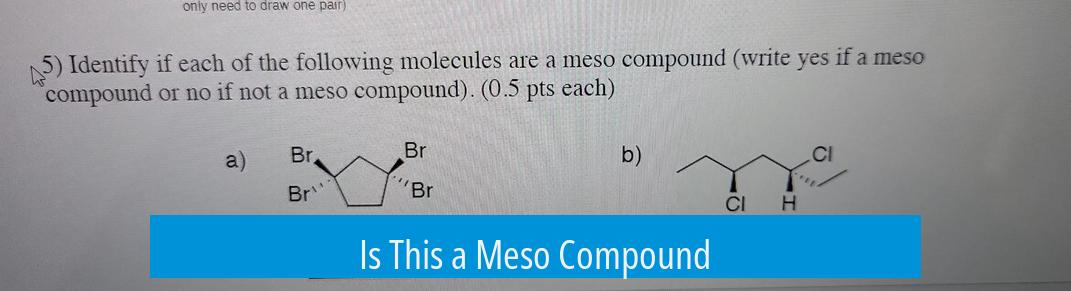
A meso compound contains chiral centers but is overall achiral due to an internal plane of symmetry. This symmetry cancels out optical activity. Identifying whether a compound is meso involves examining if it has such a plane of symmetry.
Internal Plane of Symmetry: The Key Indicator
The primary test for a meso compound is the presence of an internal plane of symmetry. This plane divides the molecule into two mirror-image halves, rendering it achiral on the whole.
- If a plane of symmetry crosses through specific atoms (often opposing carbons), the molecule is meso.
- Without this symmetry, even compounds with chiral centers remain chiral overall.
For example, a compound with two opposing bromine atoms arranged symmetrically will be meso because it allows for an internal mirror plane. If the bromines are trans-positioned and asymmetrical, the molecule lacks this symmetry and remains chiral.
Chirality vs. Meso Nature
Although meso compounds possess chiral centers, the molecule’s symmetry causes internal compensation. This means its mirror image is superimposable, unlike typical chiral molecules that have distinct enantiomers.
Molecules lacking an internal plane of symmetry maintain chirality and exhibit optical activity. Such molecules can rotate plane-polarized light, unlike meso compounds, which do not.
Comparing Two Similar Compounds
| Property | 1st Compound | 2nd Compound |
|---|---|---|
| Plane of Symmetry | Present | Absent |
| Optical Activity | None (achiral) | Active (chiral) |
| Meso Compound? | Yes | No |
Further Learning Resources
For a deeper grasp of meso compounds and chirality, the article “The Meso Trap” on Master Organic Chemistry explains these concepts well. It clarifies common confusions and details how internal symmetry affects optical activity.
Key Takeaways
- Meso compounds always have an internal plane of symmetry.
- They contain chiral centers but are overall achiral and non-optically active.
- Lack of symmetry results in chirality and optical activity.
- Examining bromine positions or substituents can help determine meso nature.
- Consult detailed resources like “The Meso Trap” for complex examples.
Is This a Meso Compound? Unlocking the Mystery of Molecular Symmetry
Wondering whether that molecule you’re staring at qualifies as a meso compound? Here’s the straight answer: a meso compound is one that has chiral centers yet remains overall achiral because it contains an internal plane of symmetry. This unique trait means its mirror image is superimposable on itself. Neat, right?
Let’s dive into why this matters and how to spot a meso compound like a pro.
Why Does the Internal Plane of Symmetry Hold the Key?
Think of a molecule as a complex sculpture. If it can be “folded” over an imagined plane so that one side mirrors the other perfectly, it has an internal plane of symmetry. This symmetry cancels out any handedness the molecule might have had.
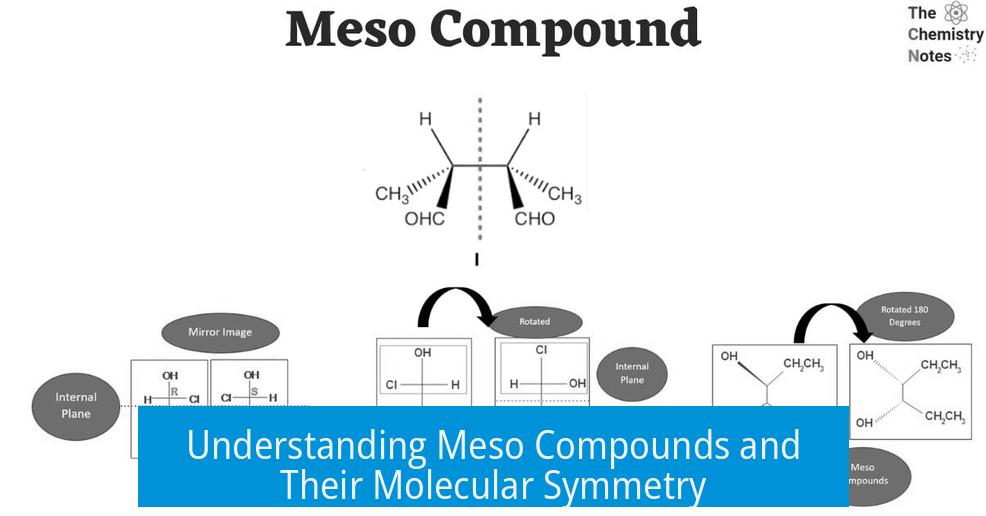
This exact idea is the holy grail for identifying meso compounds. Why? Because chirality hinges on non-superimposable mirror images. If a molecule is symmetric internally, it can’t be chiral, even if it has chiral centers. In chemistry speak, it becomes achiral overall.
Plot Twist: Meso Compounds Can Have Chiral Centers—but Don’t Be Fooled
Imagine encountering a molecule decked out with multiple chiral centers. Your first instinct might say, “This must be optically active!” But hold on. If the molecule sports that magic internal plane of symmetry, it behaves differently.
Its mirror image is not a different enantiomer but the exact same molecule again. This internal symmetry provides an “internal compensation,” canceling out optical activity. That’s the defining trait of a meso compound.
Real-Life Example: Comparing Two Compounds
| Compound | Internal Plane of Symmetry? | Chirality | Is It Meso? |
|---|---|---|---|
| First Compound | Yes — cuts through two opposing carbons | Has chiral centers but achiral overall | Yes, it’s meso |
| Second Compound | No — bromines positioned trans, no symmetry | Chiral, optically active | No, not meso |
The first compound’s plane of symmetry slices right through two opposing carbons, making it achiral overall despite those pesky chiral centers. It’s like the molecule is playing dress-up but secretly loves symmetry. This internal balance crowns it a meso compound.
The second compound might seem symmetrical, but the trans positioning of bromines ruins the party. Without that internal compensation, it’s genuinely chiral and certainly not meso.
So, How Can You Spot a Meso Compound in Your Classroom or Lab?
- Identify all chiral centers. Yes, meso compounds have them.
- Look for an internal plane of symmetry. Draw an imaginary slice where one half mirrors the other.
- Test your hypothesis by comparing the molecule with its mirror image. If they match perfectly, congrats — it’s meso!
Not convinced yet? Sometimes molecules look like they have symmetry, but subtle changes—like the position of substituents—can break it. You need a keen eye. Molecular modeling kits or software help tremendously here.
Why Does This Even Matter Beyond the Textbook?
Decoding meso compounds helps chemists predict optical activity—a huge deal in pharmaceuticals, where the handedness of molecules can mean the difference between magic medicine and a dud.
By recognizing meso compounds, chemists avoid unnecessary optical activity measurements or anticipate when a compound won’t rotate plane-polarized light, saving time and resources.
Feeling Lost? The “Meso Trap” Might Be the Hero You Need
Confused by all this talk of symmetry and chirality? Check out Master Organic Chemistry’s article on the meso trap. It’s a gem of clear explanations, perfect for avoiding common mistakes and understanding these molecules inside and out.
Summary: What Makes a Compound Meso?
- Meso compounds have chiral centers but an internal plane of symmetry.
- This symmetry cancels chirality, making them achiral overall.
- Optical activity is absent because the molecule is superimposable on its mirror image.
- Careful comparison with a non-meso compound shows how subtle differences destroy symmetry.
- Identifying meso compounds is crucial in fields like drug development.
Final Thoughts
Next time you’re handed a molecule and asked, “Is this a meso compound?” channel your inner detective. Hunt for that internal plane of symmetry. Check the placement of groups like bromines or hydroxyls carefully. Remember, a molecule with chiral centers can still be achiral if symmetry rescues it.
Here’s a parting question: Can you think of real-world molecules in pharmaceuticals or materials science where this internal symmetry shifts the entire behavior of the compound? The answer might surprise you, and that’s the fascinating beauty of chemistry.
What defines a meso compound?
A meso compound has an internal plane of symmetry that makes it achiral overall, even though it contains chiral centers. This symmetry causes internal compensation in the molecule.
How does the plane of symmetry affect chirality?
If a molecule has a plane of symmetry, it is achiral and meso. Without this symmetry, the molecule remains chiral and optically active, so it is not meso.
Can a meso compound have chiral centers?
Yes. Meso compounds have chiral centers but are achiral overall due to their symmetry, making their mirror image identical rather than an enantiomer.
Why is one compound meso and another not when both seem similar?
One compound has a clear internal plane of symmetry, making it meso. The other lacks this because of groups arranged asymmetrically, so it is chiral and non-meso.
Where can I find more detailed information on meso compounds?
The “Meso Trap” article on Master Organic Chemistry provides a clear and in-depth explanation of chirality and meso molecules. It helps clarify common confusions surrounding this topic.




Leave a Comment