How to Decide Between E1 and E2 Mechanisms
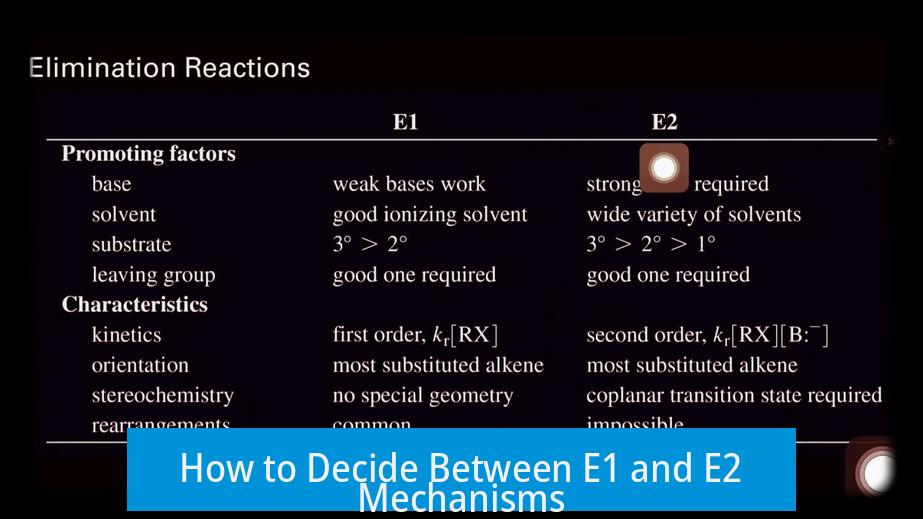
Deciding between E1 and E2 elimination mechanisms depends mainly on the base strength and the molecular geometry, particularly the presence of an anti-periplanar hydrogen. Strong bases and suitable geometric conditions favor E2, while weaker bases and different conditions often lead to E1.
1. Role of Base Strength
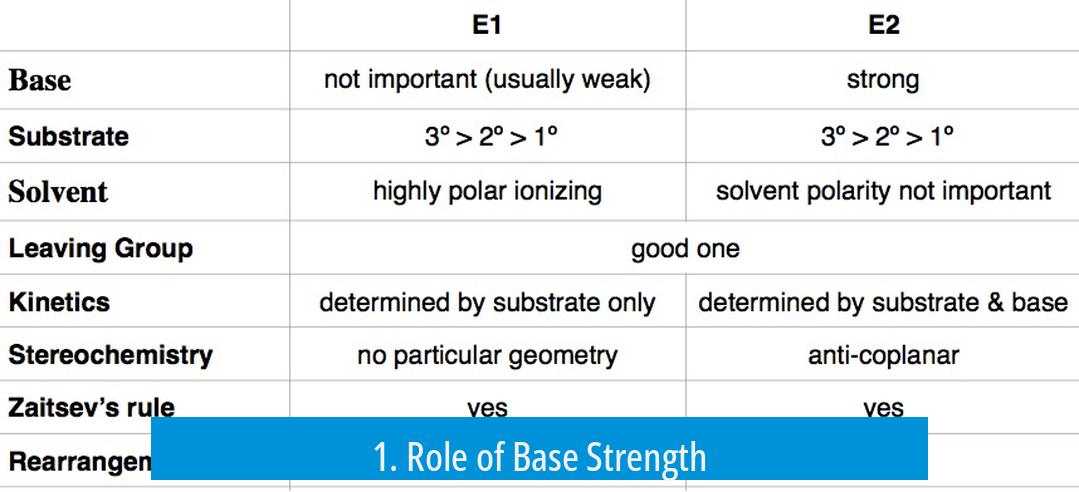
Base strength plays a critical role in determining the elimination pathway. A strong base typically favors the E2 mechanism. This is because E2 is a bimolecular, concerted process where the base abstracts a proton simultaneously as the leaving group departs.
- Strong bases such as alkoxides (e.g., NaOme) encourage E2 eliminations.
- Even though such bases can facilitate SN2 reactions, their strong basicity often causes E2 to dominate.
Conversely, E1 reactions generally proceed in the presence of weaker bases. E1 includes a carbocation intermediate and is unimolecular, so it is less sensitive to base strength but prefers conditions where carbocation formation is more favorable.
2. Importance of Stereochemical Requirements: Anti-Periplanar Hydrogen
E2 elimination requires a specific geometric arrangement. The proton that is abstracted must be anti-periplanar (180° opposite) to the leaving group.
Without this arrangement, an E2 reaction cannot proceed. This requirement is strict, as the orbital overlap during elimination demands this conformation.
- On secondary and tertiary carbons, the presence of an anti-periplanar hydrogen commonly leads to E2 elimination when paired with a strong base.
- The absence of such a hydrogen precludes E2, pushing the reaction towards E1 or other pathways.
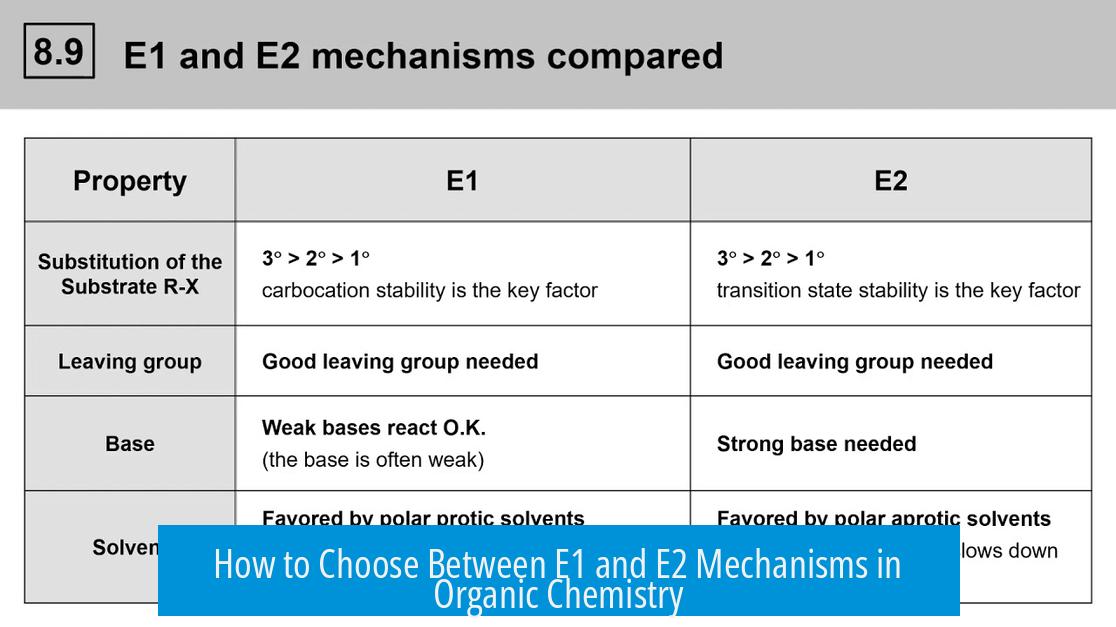
3. Summary Criteria for Choosing Between E1 and E2

| Decision Factor | E2 Mechanism | E1 Mechanism |
|---|---|---|
| Base Strength | Strong base (e.g., NaOme) | Weak base |
| Stereochemistry | Anti-periplanar hydrogen required | No geometric restriction |
| Substrate Type | Usually secondary to tertiary carbons | Prefer tertiary carbons (carbocation stability) |
| Reaction Kinetics | Concerted, bimolecular | Stepwise, unimolecular |
Key Takeaways
- Strong bases favor E2 elimination, provided an anti-periplanar hydrogen exists.
- E2 requires precise stereochemistry; no anti-periplanar proton means no E2.
- E1 occurs with weaker bases and does not need strict geometry.
- Assess both base strength and stereochemical arrangements to predict elimination pathways.
How Does One Decide Between E1 and E2?
Deciding between the E1 and E2 elimination mechanisms boils down to base strength, substrate structure, and a critical geometric demand. If the base is strong and there’s an anti-periplanar hydrogen, chances are the reaction follows the E2 pathway. Without this geometric setup and with weaker bases, E1 tends to take center stage.
Sounds simple, right? But behind that straightforward rule lies a nuanced dance of molecules and electrons that chemists have debated over (perhaps with a bit of caffeine and late-night pondering). Let’s break it down with some practical insights.
The Power of the Base: Strong Base Means E2 Excitement
Bases come in many flavors, but when it comes to elimination, their strength is the headliner. A strong base prefers the E2 pathway,
Take sodium methoxide (NaOme), for example. It’s an alkoxide base, which means it’s not only nucleophilic, but also seriously strong as a base. Despite NaOme’s ability to act in Sn2 reactions (substitution with backside attack), it favours E2 elimination—because E2 loves a strong base to snatch that proton quickly and efficiently.
This base strength rule provides a quick checkpoint: strong base + good substrate = E2 likely. But how does geometry sneak into this story?
Geometry Matters: The Hidden Requirement of Anti-Periplanar Hydrogen
E2 is a bit of a stickler when it comes to how the molecules arrange themselves. It cannot proceed without an anti-periplanar hydrogen, meaning the hydrogen that is removed and the leaving group must align opposite one another in the same plane. No proton in the anti-periplanar position? Tough luck, E2 just can’t happen.
Many learners mistakenly think this hydrogen lining-up just enhances E2. Not quite—it’s an absolute must. E2 requires this precise spatial setup to allow the base to abstract the proton as the leaving group departs simultaneously.
Imagine NaOme attacking a secondary carbon center that boasts this anti-periplanar hydrogen. The reaction practically gives E2 a spotlight.
Meanwhile, if the substrate doesn’t offer this geometric luxury, even a strong base has to pivot, possibly nudging the reaction toward E1 or other pathways.
When Does E1 Enter the Scene?
The E1 mechanism prefers to keep things chill. It doesn’t demand a strong base. Instead, it happens more with weaker bases and typically in substrates where carbocation intermediates can stabilize easily.
Unlike E2, E1 flirts with a two-step play: first, the leaving group ambles off, creating a carbocation intermediate, then a proton leaves, forming the alkene. The presence of anti-periplanar hydrogen is not a must here—geometry isn’t as strict.
That’s why in weakly basic environments, and often with more substituted carbons, E1 elimination becomes the preferred route.
But What About Substrate Structure? The Architect in This Debate
Substrate type influences the choice, too, though it’s often overshadowed by base strength and geometry.
- Methyl and primary substrates: Usually resist E1 due to instability of carbocations and lean towards Sn2 or E2, depending on base strength.
- Secondary substrates: The battleground for E1 vs E2. Strong base + anti-periplanar proton? E2 wins. Weak base, good carbocation stability? E1 may triumph.
- Tertiary substrates: Rare for E2 since they are sterically crowded—E1 often dominates if base is weak.
So, imagine the base-wielding warrior meeting a secondary carbon with an anti-periplanar hydrogen, armed with strong base like NaOme. The stage is set for E2 elimination.
Example Walkthrough: NaOme and a Secondary Carbon
NaOme is a fascinating character: strong base yet an alkoxide, with nucleophilic tendencies. It could go for Sn2 but given a secondary carbon with an anti-periplanar proton, it chooses E2. Why? Because sterics and geometry make Sn2 less probable here, and the anti-periplanar hydrogen is like an open invitation.
This helps clarify a frequent point of confusion and reinforces that elimination or substitution isn’t just about who’s stronger; it’s about how everyone is positioned for action.
Summary: Your Cheat Sheet for E1 vs E2 Decision-Making
| Condition | E2 Preferred | E1 Preferred |
|---|---|---|
| Base strength | Strong base like NaOme | Weak base |
| Geometry | Anti-periplanar hydrogen present (mandatory) | No specific geometry required |
| Substrate type | Secondary (with anti- periplanar H), primary (rare) | Tertiary or carbocation-stabilizing conditions |
| Mechanism steps | Concerted, one-step | Two-step, via carbocation intermediate |
Final Thoughts: What’s Your Reaction?
Next time you’re faced with deciding between E1 and E2, stop and ask:
- How strong is my base? Is it NaOme or something wimpy?
- Does my substrate have that crucial anti-periplanar hydrogen? Can I spot it in a Newman projection?
- How stable would a carbocation intermediate be? Will I even get one?
Answer these, and you’re well on your way to confidently predicting the pathway.
Think of it as chemistry’s version of matchmaking: fit the pieces based on strength, structure, and position, and the perfect mechanism reveals itself.
What role does base strength play in choosing between E1 and E2?
A strong base usually leads to E2 elimination. Weaker bases favor E1. Strong bases push the reaction directly to E2 unless the substrate is very unhindered.
Why is the anti-periplanar hydrogen important for E2?
Without an anti-periplanar hydrogen, E2 cannot take place. This hydrogen’s position is a strict requirement, not just a preference. It allows the base to abstract the proton while the leaving group departs.
Can a strong base also promote E1?
Strong bases generally do not favor E1 because E1 proceeds with weaker bases. If anti-periplanar hydrogen is absent, E2 is blocked, but strong bases usually still aim for elimination pathways rather than E1.
How does substrate structure influence the choice between E1 and E2?
Less hindered substrates might allow E2 even with weaker bases. Secondary carbons with anti-periplanar hydrogens typically undergo E2 when a strong base is present. Without proper geometry, E1 may dominate with weaker bases.
Why might NaOme favor E2 over SN2?
NaOme is a strong, alcholic base. It can do SN2 but favors E2 due to its basic strength and the presence of anti-periplanar hydrogens, especially on secondary carbons. SN2 is less favored in such cases.




Leave a Comment