Phenol or Alcohol? Understanding the Difference
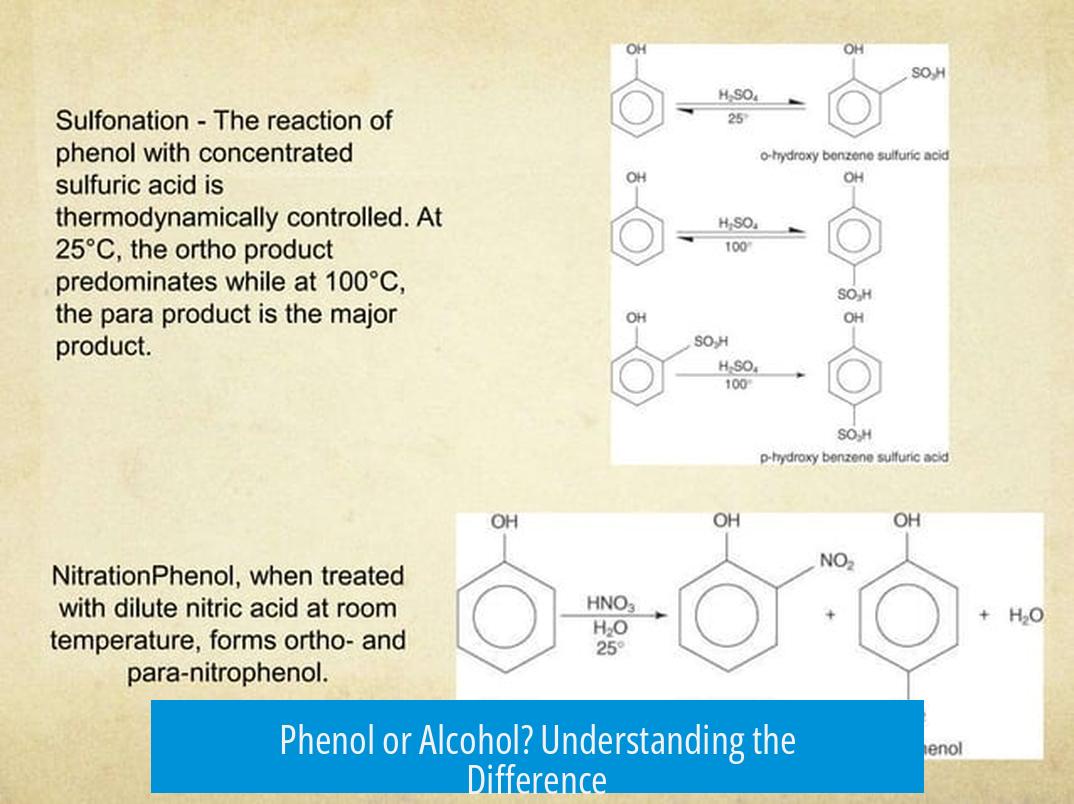
Phenol is a specific type of alcohol where the hydroxyl group (–OH) is directly attached to an aromatic phenyl ring, making it a phenolic alcohol. Alcohols broadly include compounds where –OH binds to saturated carbon atoms, while phenols have the –OH group connected to an aromatic ring.
Chemical Nature of Phenol
Phenol, chemically C6H5OH, combines features of both phenols and alcohols. It is often called a phenolic alcohol because it contains the alcohol functional group (–OH) attached specifically to a phenyl ring. This unique structure influences its chemical and physical properties distinctly from typical alcohols.
Relationship Between Phenols and Alcohols
- All phenols are alcohols due to the presence of the hydroxyl group.
- Only those alcohols with –OH directly bonded to a phenyl (aromatic) ring qualify as phenols.
- Other alcohols have –OH groups attached to saturated carbon atoms instead.
This distinction is critical in organic chemistry because phenols exhibit different reactivity patterns than regular alcohols. The presence of the aromatic ring affects acidity, solubility, and bonding.
Phenol: Molecule and Functional Group
Phenol serves as both a specific molecule and a functional group name. The IUPAC defines phenols as compounds bearing one or more hydroxyl groups attached to benzene or other aromatic (arene) rings. For example, 2-naphthol contains the phenol functional group but on a naphthalene ring instead of benzene.
The essential structural characteristic is the hydroxyl group connected directly to a phenyl ring. Without this aromatic attachment, the compound is classified simply as an alcohol.
Summary Table of Phenol vs Alcohol
| Feature | Phenol | Alcohol |
|---|---|---|
| –OH position | Attached to aromatic phenyl ring | Attached to saturated carbon atom (alkyl group) |
| Example | Phenol (C6H5OH), 2-naphthol | Ethanol (CH3CH2OH), methanol |
| Acidity | Typically more acidic due to resonance stabilization | Less acidic |
| Behavior | Different reactivity due to aromatic ring | Typical alcohol reactions (oxidation, dehydration) |
Key Points to Remember
- Phenol is a specialized alcohol with –OH attached directly to an aromatic ring.
- All phenols are alcohols, but not all alcohols are phenols.
- IUPAC classifies phenols as compounds with one or more hydroxy groups on benzene or related rings.
- The aromatic ring influences phenol’s chemical behavior and acidity.
What makes phenol different from regular alcohol?
Phenol has an –OH group attached directly to a phenyl ring, an aromatic ring. Regular alcohols have the –OH group attached to saturated carbon atoms, not aromatic rings.
Are all phenols considered alcohols?
Yes, all phenols are a special type of alcohol because they have an –OH group. However, only those alcohols with the –OH attached to an aromatic ring are phenols.
Can phenol refer to more than one thing?
Phenol is both a specific molecule and a functional group. It refers to the compound C6H5OH and to any molecule with an –OH on an aromatic ring.
How does IUPAC define phenols?
IUPAC defines phenols as compounds with one or more hydroxy (–OH) groups attached to a benzene or other arene ring, such as 2-naphthol.
Is every alcohol a phenol?
No. Only alcohols with an –OH group attached to an aromatic ring qualify as phenols. Most alcohols have –OH groups bonded to non-aromatic carbons.





Leave a Comment