Steglich Esterification with EDC: Overview and Best Practices
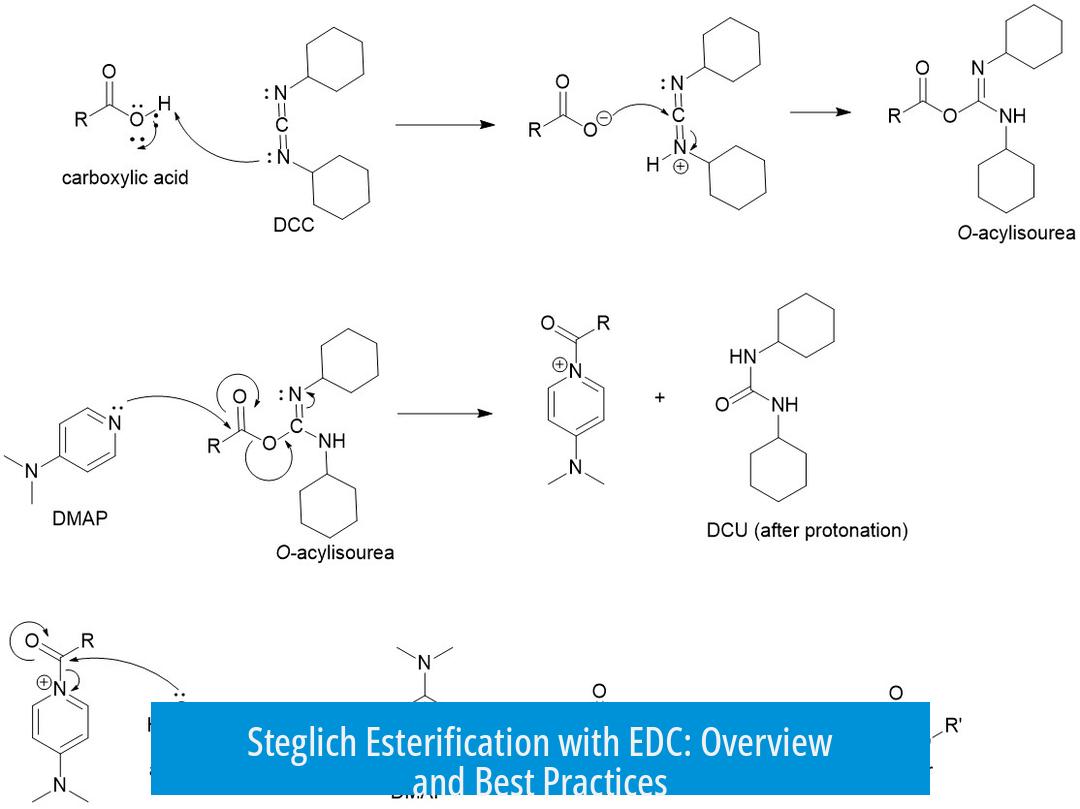
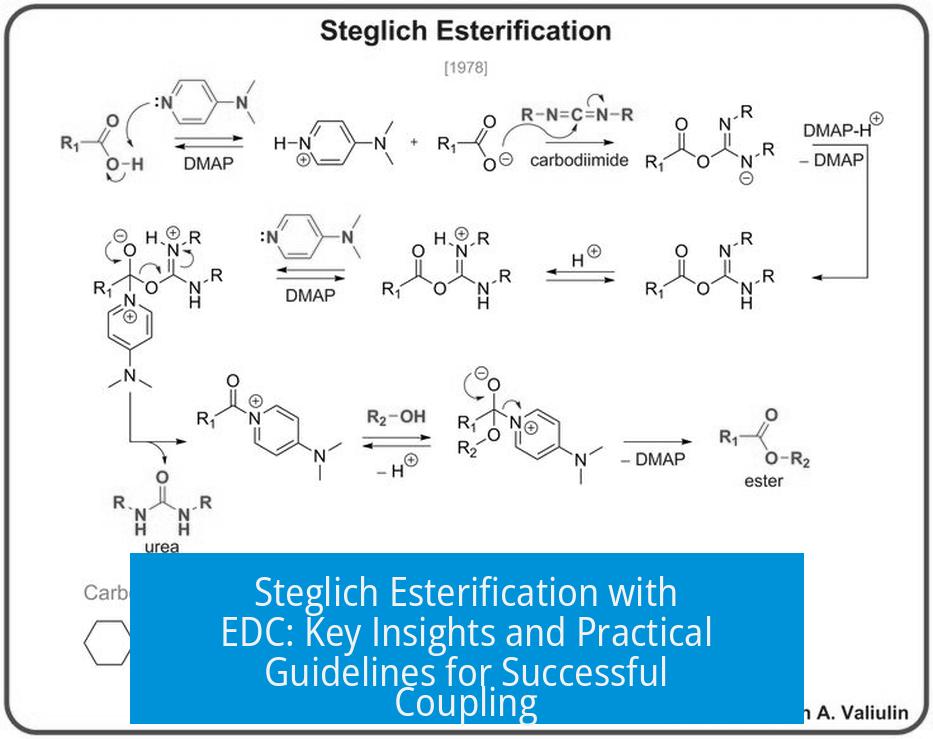
Steglich esterification with EDC is an effective method for forming esters but requires careful handling of reagents and reaction conditions. EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) is widely employed as a coupling agent in this reaction, often in place of DCC (dicyclohexylcarbodiimide), due to solubility and byproduct handling advantages. However, success depends on using bases and reaction optimization.
Role of Bases in EDC-Mediated Coupling
In Steglich esterification, adding a base is essential when using EDC or DCC. Common bases include triethylamine, DIPEA, or DMAP. They neutralize HCl released from EDC hydrochloride, preventing acid accumulation that could affect reaction efficiency. This also minimizes side reactions and ensures the amine on EDC remains reactive.
Limitations with Aliphatic Alcohols
Steglich esterification with EDC is less efficient with aliphatic alcohols. Reports show low yields due to the slower reaction rate of aliphatic alcohols, resulting in side formation of N-acyl urea byproducts. These byproducts form when the alcohol does not react rapidly enough and are water soluble, complicating purification.
Protocol Optimization for Higher Yields
Yield improvement is achievable with optimized protocols. A study published in Green Chemistry (2021) boosted yields from around 13-20% to 88%. The protocol simplifies workup and enhances ester formation efficiency through refined stoichiometry and reaction conditions.
Clarification on EDC Hydrochloride and Acid Sensitivity
- EDC hydrochloride is the neutral hydrochloride salt of the basic tertiary amine on EDC, not a chlorohydrate. It is fully compatible with esterification.
- Residual HCl does not significantly hydrolyze esters during the reaction, as the conditions are mild and room temperature is typical.
- The EDC-urea byproduct tends to retain the released HCl, preventing acidity spikes in the reaction mixture.
Summary of Practical Considerations
- Always use a base such as triethylamine or DMAP along with EDC to neutralize HCl.
- Avoid aliphatic alcohols when possible due to low ester yields and side product formation.
- Consider optimized protocols from literature to improve yields significantly.
- EDC hydrochloride salt is suitable and does not impair ester formation.
- Residual acid typically does not degrade sensitive esters under standard conditions.
Key Takeaways
- Use bases to manage HCl during EDC-based Steglich esterification.
- Steglich esterification with EDC yields are low with aliphatic alcohols due to N-acyl urea side reactions.
- Optimized reaction conditions can raise yields substantially (up to 88%).
- EDC hydrochloride is chemically suitable and does not cause acid-related ester hydrolysis.
What base should be added when using EDC in Steglich esterification?
Bases like triethylamine, DIPEA, or DMAP are added to neutralize HCl formed during the reaction. This prevents issues caused by acid buildup and improves reaction efficiency.
Why do low yields occur with aliphatic alcohols in EDC-based Steglich esterification?
Aliphatic alcohols react slowly with EDC, leading to low yields. The main side product is N-acyl urea, which forms when the alcohol fails to react fast enough.
Is there a recommended protocol to improve yields when using EDC?
Yes, an optimized protocol can increase yields dramatically, from around 13-20% up to 88%. This approach also simplifies product workup.
Can the hydrochloride salt form of EDC be used in esterification?
Yes, the hydrochloride salt of EDC is neutral and suitable for use. It is often mistakenly called chlorohydrate, but this is incorrect terminology.
Does residual HCl from EDC affect the stability of the ester product?
Residual HCl generally does not hydrolyze esters at room temperature. The HCl tends to remain bound to the urea by-product, minimizing acid impact on the ester.



Leave a Comment