Forgot to Add Ethidium Bromide in Gel Electrophoresis: What to Do

When Ethidium Bromide (EtBr) is omitted during gel preparation, the gel will run normally but DNA bands will not fluoresce under UV light. This is a frequent lab mistake that can lead to frustration when the expected bands are invisible after electrophoresis.
Identifying the Problem
EtBr intercalates with DNA allowing visualization under UV light. Without EtBr in the gel, DNA will migrate but remain unseen, causing uncertainty about the presence or size of fragments.
Many researchers experience returning after a run only to find a blank gel. This happens because the DNA has no fluorescent dye embedded during electrophoresis.
Correcting by Post-Staining the Gel

The standard correction is a post-staining step:
- Prepare a staining solution with TAE buffer (about 100 mL) and add approximately 5 μL of EtBr.
- Submerge the gel in this solution on a rocker or shaker for 25 to 30 minutes. Thicker gels or those with higher agarose concentration (e.g., 2%) may need up to 30 minutes.
- Replace the staining solution with water to destain for 6 to 15 minutes, depending on gel thickness.
This protocol allows EtBr to diffuse into the DNA within the gel, enabling bands to fluoresce under UV light.
Alternative Methods
Some suggest re-melting the gel slice and adding EtBr before re-casting. This method is more labor-intensive and may not always be practical.
Post-staining is generally preferred for convenience and efficiency.
Practical Considerations

- Handling EtBr requires caution due to its mutagenic properties.
- Ensure proper disposal of staining solutions.
- Use gloves and UV protection during imaging.
Summary of Key Points
- Omitting EtBr results in invisible DNA bands after electrophoresis.
- Post-staining the gel with EtBr in TAE buffer rescues visualization.
- Staining time depends on gel thickness and agarose concentration.
- Re-melting and re-casting the gel with EtBr is an alternative but less convenient.
- Safety precautions are essential when handling EtBr and UV light.
I Forgot to Add Ethidium Bromide in Gel: What Now?
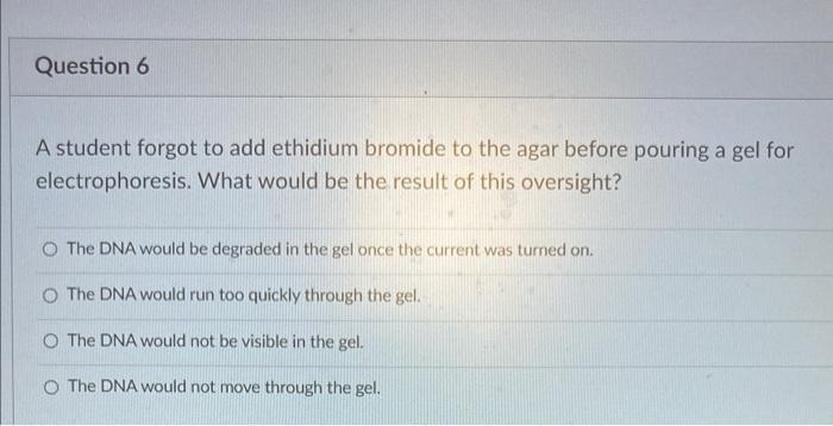
So, you just realized you forgot to add ethidium bromide (EtBr) to your agarose gel before running electrophoresis. Now what? Don’t panic. It’s a classic blunder in molecular biology labs. You run your samples, excited to check DNA bands, then… nothing. The gel looks blank, as if your DNA vanished. Been there, done that, and lived to tell the tale.
This mistake is common because EtBr is usually added directly to the gel to stain DNA, making the bands visible under UV light. Without it, your DNA remains invisible. The good news? You can fix this with a post-staining method or a creative re-melting trick.
The Surprise of a Blank Gel
Imagine going back hours later to find your gel totally blank—no DNA bands glowing purple or orange against the dark background. It stings, right? That’s the core problem of forgetting EtBr. Ethidium bromide intercalates between the bases of DNA, fluorescing under UV light, so if it’s not there, the DNA stays hidden. It’s like baking a cake but forgetting the sugar. The structure’s there but the flavor is missing.
The initial frustration is real, but the fix is worth knowing.
Post-staining: A Simple Fix That Saves the Day
The standard go-to solution is post-staining your gel. This means you didn’t add EtBr initially, but you can still expose your DNA bands by soaking the gel in an EtBr solution after electrophoresis.
Here’s how you do it:
- Prepare about 100 mL of TAE running buffer in a container.
- Add 5 μL of ethidium bromide to the buffer.
- Immerse your gel in this solution and place the container on a rocker or shaker.
- Let it stain gently for about 25 minutes.
- Swap the EtBr solution with fresh deionized water to destain for roughly 6 minutes.
This process allows EtBr to penetrate the gel and bind your DNA fragments, giving you visible bands under UV light. Easy peasy.
Note: The staining and destaining times are not set in stone. They depend on gel thickness and agarose concentration. Thick gels (like 2% agarose or more) might need longer—around 30 minutes for staining and 10-15 minutes for destaining. Don’t rush it. Staining too briefly can leave your bands faint; too long can increase background noise, making the gel look messy.
But What If You’re Feeling Adventurous?
Some folks recommend a DIY rescue: just pop the gel back into the microwave or heat source, re-melt it, and add the EtBr directly to the molten agarose. Then pour a fresh gel and re-run the experiment. It sounds drastic but can be surprisingly effective—especially if your samples are still available.
However, this method takes more time and might affect gel integrity. Plus, re-running the gel delays your results. If patience isn’t your virtue or your DNA samples are precious, post-staining is preferable.
Why Is Everyone So Invested in Fixing This?

Interestingly, the discussion about forgotten EtBr additions reveals more than lab protocols. Watching scientists brainstorm how to save a failed gel run highlights the lab camaraderie and problem-solving spirit. Even for those outside the molecular biology bubble, it’s oddly heartwarming to see people hustle together to fix something as routine as a gel staining mistake.
So if you stumbled across advice about this on social media and felt lost, join the club. Many don’t understand the jargon but appreciate the earnest efforts and support flying around. Sometimes, good vibes in science communities are just as valuable as the protocols.
Practical Tips To Avoid Forgetting EtBr
- Checklists Are Your Friends: Make a simple first-step checklist before gel prep. Include EtBr addition explicitly and tick it when done.
- Label Your Reagents: Keep your EtBr vial visible on your workspace with a sticky note reminder.
- Buddy System: If you work in a team, ask a lab mate to double-check your gel prep before electrophoresis.
- Partial Gel Staining: If you’re in a hurry, add EtBr only to the loading dye or running buffer to reduce prep errors.
Final Thoughts: Is Forgetting Ethidium Bromide the End of the World?
Absolutely not. It stings in the moment, but the post-staining solution is your best friend after the fact. It’s a scientific cliffhanger, turning a seemingly failed experiment into a successful visual. You learn, adapt, and get better at prepping gels next time.
In fact, this tiny hiccup reminds us that even experts slip up—and science is more forgiving than we think. It’s also a chance to brush up on stain techniques and understand how EtBr works. Remember, the goal is seeing your DNA bands shine under UV light, which tells you your experiment worked.
So next time you face a blank gel, don’t sigh in defeat. Grab some EtBr solution, follow the post-staining steps, and save your run from oblivion. Science is messy, but with a little patience and the right tips, you’ll turn that “I forgot” moment into a “Look what I fixed” story.
What can I do if I forgot to add Ethidium Bromide to my gel before running electrophoresis?
You can stain your gel after the run by soaking it in TAE buffer with Ethidium Bromide. Leave it on a rocker for about 25 to 30 minutes, then rinse with water to destain. The time depends on gel thickness and agarose percentage.
Is it possible to add Ethidium Bromide to the gel after it has solidified?
Yes, some suggest melting the gel again and adding Ethidium Bromide before re-casting. However, this means extra work and waiting time to remelt and solidify the gel properly.
How long should the post-staining and destaining steps take?
Post-staining typically takes 25 to 30 minutes for a thicker gel. Destaining may require 6 to 15 minutes depending on the gel’s agarose concentration. Adjust times based on gel size and thickness.
Will I still be able to see my DNA bands clearly after staining the gel post-run?
Yes, post-staining with Ethidium Bromide usually works well for visualizing DNA bands. The bands may be slightly less sharp than pre-stained gels but still visible under UV light after proper staining and destaining.
Can I avoid this problem by adding Ethidium Bromide to the running buffer instead of the gel?
Adding Ethidium Bromide to the running buffer alone is not common practice. It is usually added to the gel or used in post-staining to ensure DNA binds the dye during electrophoresis for clear band visualization.




Leave a Comment