Aufbau Principle and Hund’s Rule

The Aufbau principle and Hund’s rule guide electron configurations in atoms. The Aufbau principle describes the order electrons fill orbitals, generally from lower to higher energy. Hund’s rule governs how electrons distribute in degenerate orbitals—those with equal energy. Both rules influence atomic stability, particularly in transition metals, where exceptions and subtleties arise due to orbital energies and electron interactions.
Understanding the Aufbau Principle
The Aufbau principle means “building up” in German. It states electrons fill atomic orbitals starting with the lowest available energy levels first. Orbitals fill in sequence according to their (n + l) values, where n is the principal quantum number and l the azimuthal quantum number. Orbitals with lower sums fill before those with higher sums.
- 1s orbital fills before 2s
- 2s before 2p, then 3s, 3p, 4s, and so on
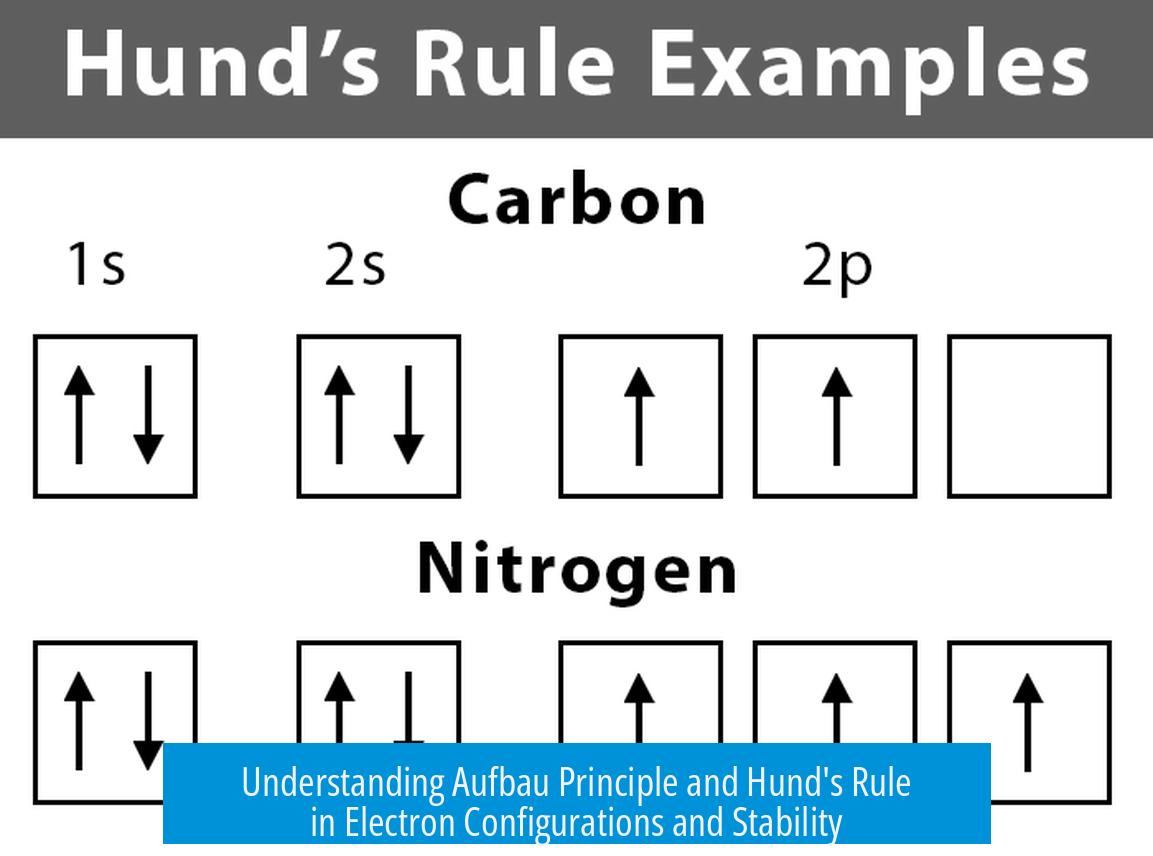
- Electrons fill orbitals singly until all degenerate orbitals have one electron each (Hund’s rule)
However, this rule is a guideline with known exceptions. Particularly, transition metals such as chromium and copper diverge from predicted fillings. Differences in orbital energies and electron-electron interactions drive these exceptions.
How Hund’s Rule Shapes Electron Configuration
Hund’s rule states electrons occupy degenerate orbitals singly, with parallel spins, before pairing occurs. This arrangement minimizes electron-electron repulsions and lowers the total energy of the atom. That is why, in a p, d, or f subshell, electrons fill one per orbital first.
For example, in the 3d subshell with five orbitals, five electrons will occupy one electron each before any pair forms. The rule increases total spin, stabilizing the atom.
Stability of Half-Filled and Full Degenerate Orbitals
Half-filled and fully filled degenerate orbitals offer extra stability. Degenerate orbitals are orbitals with the same energy level, like the five 3d orbitals. Having half or fully filled sets of these orbitals reduces total energy due to symmetry and exchange energy effects.
Electron symmetry in half-filled orbitals leads to less repulsion and more favorable energy states. This phenomenon explains why some electron configurations deviate from a strict Aufbau filling order.
| Orbital Filling | Example Configuration | Stability Reason |
|---|---|---|
| Half-filled d subshell | 3d5 (chromium: 3d54s1) | Symmetry; reduced electron repulsion |
| Fully filled d subshell | 3d10 (copper: 3d104s1) | Exchange energy maximized; closed shell stability |
These configurations lower the atom’s total energy more than other nearby configurations. For instance, chromium favors 3d54s1 instead of 3d44s2.
Energy Savings in Transition Metals
Filling or half-filling a d subshell can save more energy than filling an s orbital fully. This energy difference is because d orbitals are closer to the nucleus and have lower potential energy when symmetrical configurations appear. Transition metals also showcase electrons sometimes entering higher energy orbitals to maximize half or full subshell configurations.
Exceptions to the Aufbau Principle: Chromium and Copper
Chromium (Cr) and copper (Cu) illustrate prominent exceptions. The Aufbau principle predicts chromium to have 3d44s2, filling 4s fully before completing 3d. However, its actual configuration is 3d54s1.
This occurs because half-filling the 3d subshell lowers the atom’s energy more than filling 4s completely before 3d. This half-filled configuration maximizes exchange energy and orbital symmetry.
Copper shows a similar exception. Instead of 3d94s2, copper favors 3d104s1. A fully filled 3d subshell stabilizes the atom better than a partially filled d subshell paired with a fully filled 4s.
The difference in electron count arrangement affects combinatorial possibilities. For chromium’s 3d5, there’s a unique symmetrical arrangement. For 3d4, multiple arrangements exist, indicating less stability.
Explanation with Electron Arrangements
- Chromium’s 3d54s1: Electrons arranged uniquely in degenerate orbitals, minimizing energy.
- Chromium’s 3d44s2: Multiple ways to arrange electrons increase instability.
This symmetry principle follows from Hund’s rule coupled with orbital energy considerations.
Orbital Energy Ordering and the n+l Rule
The n+l rule predicts orbital filling order by summing principal (n) and azimuthal (l) quantum numbers. The orbital with smaller n+l generally fills first. If two orbitals have the same n+l, the one with smaller n fills first.
| Orbital | n | l | n + l | Filling Priority |
|---|---|---|---|---|
| 4s | 4 | 0 | 4 | Filled before 3d |
| 3d | 3 | 2 | 5 | Filled after 4s |
However, from scandium (Sc) onward, deviations appear. Despite 4s filling before 3d in neutral atoms, the 3d orbitals have lower energy once electrons have populated them. Hence, when ions form, electrons are removed from 4s before 3d.
This nuance explains the difference between filling order for neutral atoms and electron removal during ionization.
Hund’s Rule Applied to Degenerate Orbitals
Hund’s rule maximizes total electron spin by placing electrons in separate degenerate orbitals with parallel spins. This reduces electron repulsion by maximizing the exchange interaction.
For example, in chromium’s 3d5 subshell, each electron occupies a separate d orbital with aligned spins. This arrangement lowers total energy compared to any paired distribution within fewer orbitals.
The combinatorial options for electron arrangement differ based on half-filled vs. partially filled subshells. Half-filled subshells present lower energy due to symmetric electron distribution, validating Hund’s rule experimentally.
Consequences for Electron Configurations
- Maximizing unpaired electrons increases stability in degenerate orbitals
- Electrons avoid pairing until necessary, mitigating repulsive forces
- Atomic configurations deviate from naive Aufbau predictions when the increased stability of half-full or full subshells makes a higher energy orbital filling preferred
Transition Metals: Symmetry, Stability, and Energy Minimization
Electron configurations in transition metals involve complex interplays between energy levels. A major source of stability is symmetry maximization in electron distribution within orbitals.
Symmetrical configurations minimize repulsion and maximize exchange energy, resulting in reduced atom energy. Hence, full and half-full d subshells are particularly stable, sometimes overriding the order predicted purely by energy calculations for isolated orbitals.
This explains why transition metals can have rather unpredictable electron configurations.
Electrons and Closer Orbitals to the Nucleus
In transition metals, d orbitals often lie closer to the nucleus and experience less shielding compared to s orbitals in the same principal energy level. Electrons occupying these orbitals experience lower potential energy.
As such, filling or half-filling d orbitals can contribute more significantly to atom stability than fully occupying an s orbital with a slightly higher potential energy.
Exceptions in Heavy Elements: Tungsten and the 6th Period
Heavy elements such as tungsten present more complicated electron configurations. In tungsten, observed electron distribution differs from simple Hund and Aufbau predictions. For example, tungsten’s configuration is [Xe]4f145d46s2, showing occupancy patterns that differ from Hund’s rule for the 5d subshell.
Such exceptions arise due to subtle effects:
- Relativistic effects influencing electron masses and velocities
- Electron-electron interactions becoming more complex in larger atoms
- Close energy values between subshells affected by nuclear charge and shielding
These factors cause deviations from simple filling rules that hold better for lighter elements.
Summary: Key Points on Aufbau Principle and Hund’s Rule
- Aufbau Principle guides electron filling from lower to higher energy orbitals, but exceptions exist.
- Hund’s Rule requires electrons to occupy degenerate orbitals singly before pairing, maximizing total spin and lowering energy.
- Half-filled and fully filled degenerate orbitals confer extra stability via symmetry and exchange energy.
- Chromium and copper demonstrate Aufbau exceptions due to stability from half/full d subshell filling.
- From scandium, 3d orbitals have lower energy than 4s, affecting electron filling and removal order.
- Transition metals show complex stability patterns arising from orbital energies and electron interactions.
- Heavy elements such as tungsten deviate further due to relativistic and electron correlation effects.
What makes half-filled and fully filled degenerate orbitals more stable?
Half-filled and fully filled degenerate orbitals have lower energy due to electron symmetry. This symmetry decreases repulsion, resulting in greater stability than uneven electron distributions in degenerate orbitals.
Why do Chromium and Copper have exceptions to the Aufbau principle?
They favor configurations with half-filled or fully filled d subshells because these arrangements are more stable. For example, Chromium prefers 3d⁵4s¹ instead of 3d⁴4s² to maximize stability via electron arrangement.
How does Hund’s rule affect electron filling in degenerate orbitals?
Hund’s rule directs electrons to fill orbitals singly with parallel spins first. This maximizes total spin and reduces electron repulsion, leading to lower energy and more stable atomic configurations.
Why do 4s and 3d orbitals sometimes defy the n+l rule?
From scandium onward, 3d orbitals lie lower in energy than 4s, so electrons fill 4s first but are removed from 4s before 3d during ionization. The n+l rule is a guide but not absolute, especially for transition metals.
What causes exceptions to Hund’s rule and the Aufbau principle in heavy elements like tungsten?
In 6th period elements, factors such as electron-electron interactions, relativistic effects, and subtle energy differences disrupt normal filling. These effects cause deviations from expected Hund’s and Aufbau patterns.




Leave a Comment