Is There a Way to Reclaim Oxygen from Carbon Dioxide Through a Process That Could Last Hours with a Nontoxic Byproduct?
Yes, it is possible to reclaim oxygen from carbon dioxide through certain chemical and electrochemical processes. However, these processes either produce toxic byproducts or require significant energy input. One notable method involves lithium peroxide, which reacts with CO2 to release oxygen and produces relatively nontoxic lithium carbonate. While this can sustain oxygen reclamation for hours, recycling the lithium peroxide cartridge demands additional chemical steps. Alternative methods like electrolytic reduction produce oxygen but yield toxic carbon monoxide as a byproduct and need substantial energy. Thus, a long-lasting, low-toxicity oxygen reclamation system from CO2 exists but faces practical and energy challenges.
Existing Technologies for Managing CO2 and Supplying Oxygen

Rebreathers and CO2 Absorption
Rebreathers do not convert CO2 back to oxygen. They function by absorbing the carbon dioxide exhaled by the user. This absorption prevents CO2 buildup that causes acidosis or hypercapnia and allows the remaining oxygen in the breathing loop to be reused. However, rebreathers neither generate oxygen nor reduce CO2 concentration beyond absorption capability. This limits their duration and oxygen reclamation capability.
Submarine Systems: Electrolysis and CO2 Scrubbing
Submarines generate oxygen by electrolyzing seawater. This process splits water molecules into oxygen and hydrogen gases. The hydrogen is discarded, while oxygen is replenished in the air supply. Meanwhile, carbon dioxide is removed using scrubbing agents or absorption on materials. This method effectively separates oxygen from water rather than recycled CO2, meaning it does not reclaim oxygen directly from carbon dioxide molecules.

Zeolites for Gas Adsorption
Zeolites are porous materials capable of selectively adsorbing gases like CO2. They trap carbon dioxide molecules by fitting them within their crystal structure and release them upon pressure changes. This process aids in CO2 removal but does not chemically convert CO2 into oxygen or other species, hence does not reclaim oxygen.
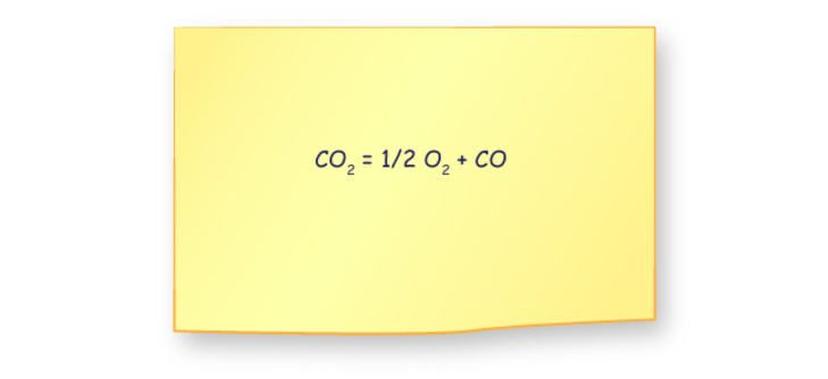
Chemical and Electrolytic Conversion of CO2 into Oxygen
Electrolytic Reduction of Carbon Dioxide
Electrochemical cells can split carbon dioxide molecules to produce oxygen and other compounds. For example, some membrane electrode assemblies convert two CO2 molecules into two molecules of carbon monoxide (CO) and one molecule of oxygen (O2). The reaction can be summarized:
2 CO2 → 2 CO + O2
This process recovers only half of the oxygen atoms present in the original CO2. Additionally, the CO generated is a toxic gas requiring careful management or removal, which complicates its use in confined environments.
Energy Requirements and Feasibility
The electrochemical reduction of CO2 into O2 demands substantial energy input. Since this reaction reverses combustion, which releases energy, it is inherently energy unfavorable. The process is sustainable only with cheap and renewable energy sources such as solar or wind power. Due to high energy consumption, practical deployment currently remains limited to research and specialized applications.
Future Prospects and Sci-Fi Concepts
Advanced catalysts or genetically engineered microorganisms might make CO2-to-oxygen conversion more efficient and manageable. NASA has funded research to develop electrolyzers capable of producing oxygen for astronauts on Mars by converting CO2 from the atmosphere while generating useful organic compounds like ethanol. These methods remain in experimental or developmental stages.
Lithium Peroxide-Based Rebreather and Oxygen Reclamation
Chemical Reaction of Lithium Peroxide with CO2
Lithium peroxide (Li2O2) reacts with carbon dioxide to regenerate oxygen and form lithium carbonate (Li2CO3). The reaction is:
2 Li2O2 + 2 CO2 → 2 Li2CO3 + O2
This reaction directly releases molecular oxygen and sequesters CO2 as lithium carbonate, a relatively nontoxic solid. It provides efficient oxygen reclamation suitable for closed environments and life support systems where safety and long-lasting activity are critical.
Recycling Lithium Peroxide Cartridges
To maintain oxygen generation capacity, the lithium carbonate produced can be subjected to thermal and chemical steps to regenerate lithium peroxide:
- Heat lithium carbonate to decompose it into lithium oxide (Li2O) and CO2.
- React lithium oxide with water to form lithium hydroxide (LiOH).
- Treat lithium hydroxide with hydrogen peroxide (H2O2) to produce lithium hydroperoxide (LiOOH).
- Heat lithium hydroperoxide to regenerate lithium peroxide (Li2O2) cartridge.
This multi-step recycling is energy- and equipment-intensive but allows cartridge reuse, minimizing waste and exposure to toxic byproducts.
Limitations and Challenges for Oxygen Reclamation Systems
Toxic Byproducts from Chemical Conversion
Many chemical methods for direct reclamation of oxygen from CO2 generate toxic byproducts such as carbon monoxide or other reactive carbon species. These toxic products pose health risks and require complex handling procedures, limiting their application in manned environments without extensive safety controls.
Energy Intensity and Practical Scale
Processes that chemically split CO2 into O2 and carbon species require significant energy, making them economically and practically challenging except where abundant, clean energy exists. In small closed systems like spacesuits, aggressive chemical processes are unsafe. Technologies like lithium peroxide rebreathers balance safety and efficacy but need consumable cartridges and recycling capabilities.
Practicality of Long-Lasting Nontoxic Oxygen Reclamation Systems
Long-lasting and nontoxic oxygen reclamation directly from CO2 is theoretically possible but currently constrained by:
- High energy requirements for electrochemical or thermal processes.
- Production of toxic carbon byproducts in many chemical methods.
- Need for consumable or recyclable chemical reagents (e.g., lithium peroxide cartridges).
- Complexity and bulk of recycling protocols limiting compact system design.
Biological approaches, such as photosynthesis by algae or engineered microbes, effectively recycle CO2 to oxygen using sunlight energy, but require space, light, and time—making them less suitable for rapid or compact applications.
Key Takeaways
- Reclaiming oxygen from carbon dioxide is possible through chemical or electrochemical processes but commonly yields toxic byproducts.
- Lithium peroxide reacts with CO2 to produce oxygen and lithium carbonate, a relatively safe byproduct, allowing oxygen recovery over hours.
- Recycling lithium peroxide cartridges involves multi-step chemical reactions needing energy and equipment.
- Electrolytic CO2 reduction produces oxygen and carbon monoxide, requiring large energy input and CO management.
- Current technologies for closed environments often focus on CO2 scrubbing and oxygen supply from water electrolysis rather than direct CO2-to-O2 conversion.
- Long-lasting, nontoxic oxygen reclamation from CO2 remains challenging due to energy, safety, and recycling requirements.
Is There a Way to Reclaim Oxygen from Carbon Dioxide Through a Process That Could Last Hours with a Nontoxic Byproduct?
In short: Yes, but with caveats. There are methods to reclaim oxygen from carbon dioxide, lasting hours and producing mostly nontoxic byproducts, yet they come with trade-offs involving energy input, byproducts’ nature, and practicality—especially in closed, small spaces like spacesuits.
Let’s unpack this fascinating question layer by layer, examining existing tech, emerging science, and the challenges that stand in the way.
First, What’s Happening With CO2 and Oxygen Now?
Imagine you’re underwater, or even in a spacecraft. You breathe out carbon dioxide (CO2), which can become toxic in enclosed spaces. How do these environments stay breathable?
One simple solution is the rebreather. It doesn’t convert CO2 back into oxygen—instead, it traps CO2 to keep you from poisoning yourself. Think of it like holding your breath longer by just removing the bad stuff. But does that solve the oxygen problem? No, it just makes what oxygen remains last longer. That’s recycling, not reclaiming.
Submarines take a more advanced route. They don’t rely on magic; they use nuclear power to split seawater into oxygen and hydrogen. The oxygen refreshes the breathing air.
Electrolysis—that’s the word—breaks water molecules into oxygen (which goes into the air) and hydrogen (which is discarded). Meanwhile, the CO2 in the cabin air is scrubbed separately, often with chemical scrubbers.
So, submarines manage oxygen and CO2 separately and don’t attempt to get oxygen straight from CO2 itself.
Can We Directly Convert CO2 to Oxygen?
This is where it gets interesting. Chemically ripping oxygen atoms off CO2 is tough. It’s an energy-hungry reverse of burning carbon fuels—a process nature does unthinkably well via plants but we struggle to imitate.
The key reactions scientists look at include electrolytic reduction:
- CO2 + 2e− + 2H+ → CO + H2O
- 2H2O → O2 + 4H+ + 4e−
- Net result: 2 CO2 → 2 CO + O2
You get oxygen, yes, but also carbon monoxide (CO). That’s toxic stuff, so you must carefully separate it. Plus, only about half the oxygen atoms from the CO2 get reclaimed as O2; the rest tag along as CO.
Crucially, this process is energy intensive. It’s not just flipping a switch. Without a cheap, renewable power source (like solar or advanced batteries), it’s impractical.
Could future tech fix this? Sci-fi visions dream of catalysts or engineered microbes converting CO2 into pure oxygen and harmless carbon pellets. NASA even funds projects to develop electrolyzers to turn Mars’ CO2 atmosphere into oxygen and ethanol. But these methods remain experimental or futuristic.
Lithium Peroxide: A Close-to-Real-World Hero?
Here’s a neat chemical trick known since the mid-20th century: lithium peroxide (Li2O2) reacts with CO2 to generate oxygen and lithium carbonate.
The reaction looks like this:
| Reactants | Products |
|---|---|
| 2 Li2O2 + 2 CO2 | 2 Li2CO3 + O2 |
This reaction happens in some advanced rebreathers. It grabs CO2, dumps oxygen back into the air, and forms lithium carbonate—a stable, nontoxic solid byproduct.
Can you keep the cycle going? Lithium carbonate can be heated to release CO2 and produce lithium oxide. Then through a series of chemical steps involving water and hydrogen peroxide, you can regenerate lithium peroxide. This recycling allows for longer use and less waste.
Sounds great—but there’s a catch: the recycling process needs heat and chemicals to work, and it takes time and careful handling.
Challenges: Energy, Scale, and Safety
It’s easy to say “reclaim oxygen from CO2.” But:
- The process often produces toxic byproducts like carbon monoxide.
- It requires lots of energy, which must be cheap or renewable to be sustainable.
- At small scales (spacesuits or closed cabins), chemical reactions can be dangerous or violent.
- Handling lithium compounds demands specialized knowledge and equipment.
That means for tiny closed environments, scrubbing CO2 and separately generating oxygen (via electrolysis) remains the standard, most practical approach.
So, What’s The Best Way Forward?
Right now, for missions like underwater dives or space exploration, a combination of CO2 scrubbing and external oxygen generation is the real deal. This combo keeps air breathable without mysterious, dangerous byproducts. Lithium peroxide cartridges offer oxygen with a nontoxic byproduct, and recycling the cartridge is possible but complex.
Will there ever be a perfect device that turns your exhaled CO2 back into oxygen for hours on end, with simple, safe byproducts? Possibly, yes—but only with breakthroughs in:
- Efficient, low-energy catalysis or biological converters.
- Nanotechnology filters that separate gases cleanly.
- Renewable energy sources powering the whole show.
Imagine a future astronaut tossing out harmless carbon pellets and breathing clean air recycled perfectly—that’s sci-fi now, it might become science fact soon.
FAQs and Recommendations
- Why not just use plants? Plants do recycle CO2 into oxygen but need light, water, and time. In confined, dark environments like space, they’re slow and bulky.
- Could I build a CO2-to-O2 recycler at home? Not safely or practically. The processes require controlled chemical reactions, energy, and handling hazardous materials.
- What about toxic byproducts? Handling CO and other gases means strong safety measures and separation tech, adding complexity.
The Bottom Line
Yes, reclaiming oxygen from CO2 with a nontoxic byproduct over hours is possible but challenging. Current systems prefer scrubbing CO2 and generating oxygen separately. Lithium peroxide offers a semi-cyclic solution with manageable byproducts but demands recycling chemistry. Electrolytic reduction is promising yet energy-hungry and produces toxic CO.
For now, if you’re planning an underwater expedition or spacewalk, rely on tried-and-tested scrubbers and oxygen supplies. But keep an eye on advancements—tomorrow’s tech could revolutionize breathable air regeneration and make sci-fi oxygen recyclers a reality. Who wouldn’t want that?
Can Lithium Peroxide be used to reclaim oxygen from carbon dioxide safely?
Yes. Lithium peroxide reacts with CO2 to form lithium carbonate and oxygen. This process produces oxygen without toxic byproducts.
Is the lithium peroxide cartridge reusable or recyclable?
Yes, through heating and chemical steps, lithium carbonate can be converted back to lithium peroxide. This recycling process helps maintain the system over hours or longer.
Are there chemical methods that convert CO2 to oxygen without toxic byproducts?
Most chemical conversions produce toxic byproducts. Lithium peroxide is an exception, making it one of the few safe options at small scales.
What are the energy demands of converting CO2 to oxygen?
Electrolytic conversion is very energy-intensive and unfavorable. It relies on cheap energy sources like sunlight or renewables to be practical over hours.
Why do rebreathers not truly reclaim oxygen from carbon dioxide?
Rebreathers absorb CO2 but do not convert it back to oxygen. They extend breathable air but do not produce new oxygen from CO2.


Leave a Comment