Have you ever wondered why atoms have different electron configurations? We’ve all heard of the 2 8 8 18 rule in chemistry, but what does it mean? This rule is all about the arrangement of electrons in various shells, sub-shells and orbitals in an atom. But how did this arrangement come to be? Why is it so important?
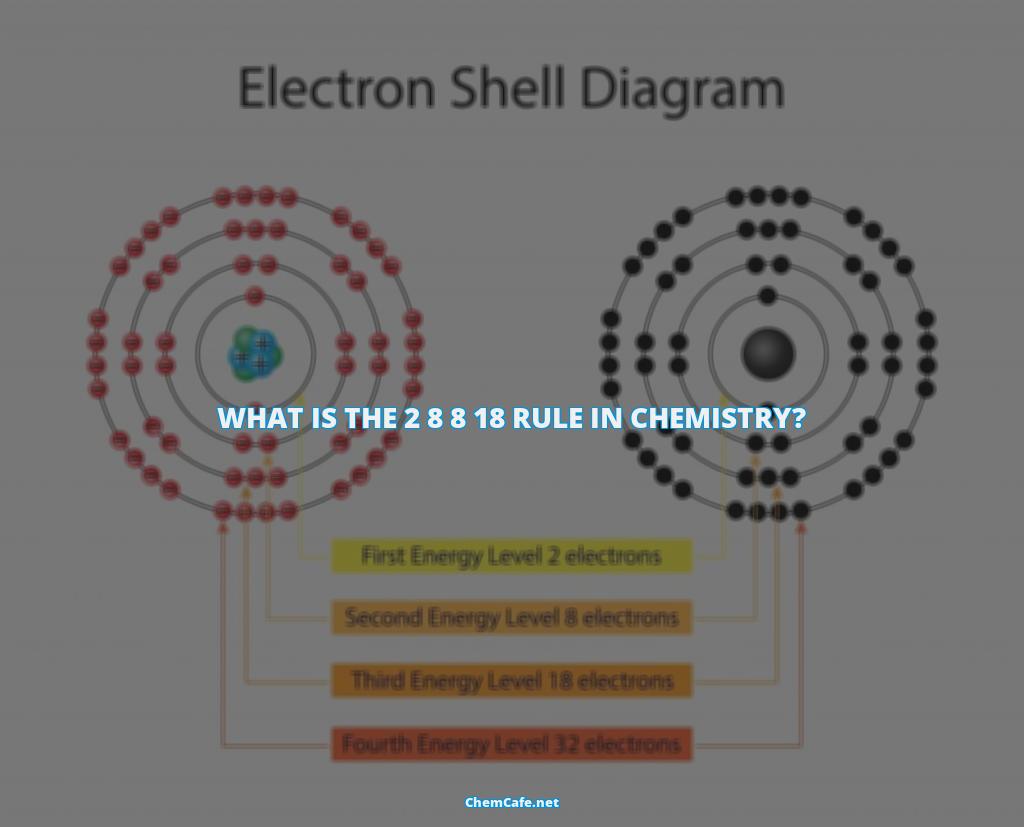
The 2 8 8 18 rule is an easy way to understand the electron configuration of an atom. An atom is made up of protons, neutrons, and electrons. The protons and neutrons form the nucleus, while the electrons are arranged in shells around the nucleus. Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on.
The 2 8 8 18 rule states that the first shell is filled with two electrons, the second is filled with eight electrons, and the third is filled with eight. This is why sodium (Na) and magnesium (Mg) have a few extra electrons. This rule explains why some atoms have more electrons than others. For example, oxygen (O) has eight electrons in its outermost shell, while neon (Ne) has ten electrons in its outermost shell.
The 2 8 8 18 rule can also be used to predict how atoms will react with each other. By understanding the electron configurations of atoms, scientists can predict how they will bond with each other. This helps them understand the properties of different elements, and how they interact with each other.
In conclusion, the 2 8 8 18 rule is a great way to understand the electron configurations of atoms. It is an essential tool for chemists and physicists, who rely on this rule to understand how atoms interact with each other. This rule explains why some atoms have more electrons than others, and how they are arranged in shells around the nucleus.
What is the 2 8 8 18 rule in chemistry?
The 2 8 8 18 rule in chemistry is a way of organizing the electrons of an atom. It is also known as the electron configuration rule and is based on the number of electrons in each shell of an atom. This rule helps chemists better understand the structure of atoms and how they interact with each other.
Atoms have a nucleus, which is surrounded by a number of electrons. The number of electrons in each shell is determined by the atomic number of the element, which is the number of protons in the nucleus. According to the 2 8 8 18 rule, the first shell of an atom can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on.
What is the 2 8 8 electron rule?
The 2 8 8 electron rule is a simple way of remembering the number of electrons that can fit into each shell of an atom. It states that the first shell can hold two electrons, the second shell can hold eight, and the third shell can hold eighteen. This rule applies to all atoms with atomic numbers between 1 and 18.
Why is the electron configuration 2 8 8 18?
The electron configuration 2 8 8 18 is the most common way of arranging the electrons of an atom. This configuration is based on the number of electrons in each shell of the atom. It is written as 2, 8, 8, 18, 18, 32.
The first two shells are filled with two and eight electrons, respectively. The third shell is filled with eight electrons, and the fourth and fifth shells are filled with eighteen electrons each. This arrangement of electrons is known as the 2 8 8 18 rule in chemistry and is the most common way of arranging electrons in an atom.
What is the 2 8 8 18 rule in chemistry used for?
The 2 8 8 18 rule in chemistry is used to understand the structure of atoms and how they interact with each other. It is also used to predict the chemical properties of elements and compounds. By understanding the arrangement of electrons in an atom, chemists can better predict how elements will react with each other.
The 2 8 8 18 rule also helps chemists determine the type of bond that will form between two atoms. Knowing the arrangement of electrons in an atom can help chemists determine whether the bond between two atoms will be covalent, ionic, or metallic.
The 2 8 8 18 rule in chemistry is a way of organizing the electrons of an atom. It is based on the number of electrons in each shell of an atom and helps chemists better understand the structure of atoms and how they interact with each other. It is also used to predict the chemical properties of elements and compounds, as well as the type of bond that will form between two atoms.
What is 8 electron rule?
The 8 electron rule is an important concept in chemistry that explains why atoms form bonds and molecules with certain structures. This rule states that atoms tend to have eight electrons in their valence shell, which is the outermost shell of an atom. This is because atoms with fewer than eight electrons in their valence shell tend to react and form more stable compounds.
The Valence Shell
The valence shell is the outermost shell of an atom, and it contains electrons that are available for bonding. The number of valence electrons can vary, but the 8 electron rule states that atoms tend to prefer having 8 electrons in the valence shell. This is because 8 electrons create a more stable configuration that is more likely to be successful in forming bonds.
The Octet Rule
The 8 electron rule is also known as the octet rule, which refers to the tendency of atoms to prefer to have eight electrons in the valence shell. This rule applies to all atoms except for those in the transition metal or inner-transition metal blocks, as these elements have a different electron configuration. For elements outside of these blocks, an octet configuration is achieved when the atom has an electron configuration ending with (s^2p^6).
Abegg’s Rule
In 1904, Richard Abegg formulated what is now known as Abegg’s rule, which states that the difference between the maximum positive and negative valences of an element is frequently eight. This rule is based on the observation that atoms tend to form bonds with other atoms in order to achieve an octet configuration. By forming bonds, atoms can exchange electrons in order to achieve a more favorable configuration.
Importance of the 8 Electron Rule
The 8 electron rule is an important concept in chemistry that explains why atoms form bonds and molecules with certain structures. By understanding the 8 electron rule, chemists can predict the structure of molecules and the formation of chemical bonds. This rule is also important for understanding the stability of compounds, as molecules with an octet configuration tend to be more stable than those with fewer electrons in the valence shell.
In conclusion, the 8 electron rule is an important concept in chemistry that explains why atoms form bonds and molecules with certain structures. This rule states that atoms tend to have eight electrons in their valence shell, which is the outermost shell of an atom. This is because atoms with fewer than eight electrons in their valence shell tend to react and form more stable compounds. The 8 electron rule is also known as the octet rule, and it applies to all atoms except for those in the transition metal or inner-transition metal blocks. By understanding the 8 electron rule, chemists can predict the structure of molecules and the formation of chemical bonds.
What is the 2 8 8 rule in chemistry?
The 2 8 8 rule in chemistry is a rule that states that elements in the second period of the periodic table will fill their outer shells with 8 electrons. This rule is also known as the octet rule because it states that the element must have 8 electrons in its outer shell. The rule is a useful tool for predicting the stability and reactivity of an element.
The 2 8 8 rule applies to the elements in the second period of the periodic table, which includes carbon, nitrogen, oxygen, fluorine, sodium, and magnesium. The rule states that the outer shell of these elements should have 8 electrons. This ensures that the outer shell of the element is filled and stable. This is important because it makes the element more stable and less reactive.
What is the Origin of 2 8 8 Rule?
The 2 8 8 rule was first proposed by Gilbert N. Lewis in 1916. Lewis proposed the octet rule as a way to explain the stability of elements in the second period of the periodic table. He observed that elements in this period often had 8 electrons in their outer shells, and this created a stable configuration.
How does the 2 8 8 Rule Work?
The 2 8 8 rule works by having atoms try to achieve a full outer shell of electrons. This is done by gaining, losing, or sharing electrons with other atoms. The octet rule states that atoms will try to gain or lose electrons until they have 8 electrons in their outer shell. This makes the atom more stable and less reactive.
What are the Exceptions to the 2 8 8 Rule?
The 2 8 8 rule is a useful tool, but it does have some exceptions. For example, elements in the third period of the periodic table (such as sulfur and phosphorus) do not follow the octet rule. This is because these elements have more than 8 electrons in their outer shells.
In addition, some elements in the second period of the periodic table do not follow the octet rule. For example, elements such as beryllium, lithium, and boron have fewer than 8 electrons in their outer shells. These elements are known as “inert gases” because they are generally less reactive than other elements.
The 2 8 8 rule in chemistry is a useful tool for predicting the stability and reactivity of elements in the second period of the periodic table. The rule states that these elements should have 8 electrons in their outer shells. This ensures that the element is stable and less reactive. However, there are some exceptions to the rule, such as elements in the third period of the periodic table and elements such as beryllium, lithium, and boron.
What is the rule of 8 in atoms?
Atoms tend to form stable molecules when their outermost shell is full of electrons. This is known as the octet rule and states that atoms will typically have eight electrons in their valence shell. This rule applies to most of the elements that are important for biological processes, including carbon, nitrogen, oxygen, and hydrogen.
What is 8 electron rule?
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. When discussing the octet rule, we do not consider d or f electrons.
What is the rule for 8 valence electrons?
In general, atoms are most stable, least reactive, when their outermost electron shell is full. Most of the elements important in biology need eight electrons in their outermost shell in order to be stable, and this rule of thumb is known as the octet rule.
Do all elements need 8 electrons?
While most atoms obey the duet and octet rules, there are some exceptions. For example, elements such as boron or beryllium often form compounds in which the central atom is surrounded by fewer than eight electrons (e.g., BF₃ or BeH₂).
What are the 3 exceptions to the octet rule?
However, there are three general exceptions to the octet rule: Molecules, such as NO, with an odd number of electrons; Molecules in which one or more atoms possess more than eight electrons, such as SF6; and Molecules in which two or more atoms share electrons, such as H2O.
In the case of NO, the nitrogen atom has five electrons in its outer shell and the oxygen atom has six electrons. This arrangement is more stable than if each atom had eight electrons in its outer shell.
In the case of SF6, the sulfur atom has six electrons in its outer shell and the six fluorine atoms have eight electrons each. This arrangement is more stable than if each atom had eight electrons in its outer shell.
Finally, in the case of H2O, the two hydrogen atoms share two electrons with the oxygen atom. This arrangement is more stable than if each atom had eight electrons in its outer shell.
In conclusion, the octet rule states that atoms tend to form stable molecules when their outermost shell is full of electrons. However, there are three exceptions to this rule, namely molecules with an odd number of electrons, molecules with more than eight electrons, and molecules in which two or more atoms share electrons. Knowing and understanding these exceptions can help chemists and biologists better understand the structure and behavior of molecules.
Why are there 8 electrons in a shell?
Atoms are composed of particles called protons, neutrons, and electrons. Electrons are found in shells, which are concentric circles surrounding the nucleus of the atom. In general, atoms are most stable, least reactive, when their outermost electron shell is full. Most of the elements important in biology need eight electrons in their outermost shell in order to be stable, and this rule of thumb is known as the octet rule.
The First Shell and the Octet Rule
The first shell is filled with 2 electrons, the second is filled with 8 electrons, and the third is filled with 8. You can see that sodium (Na) and magnesium (Mg) have a couple of extra electrons. But why can the outermost shell have more than 8 electrons?
When we observe the periodic table carefully we find that d and f electrons are added is (n−1) and (n−2) shells and only s and p electrons are added in nth shell. So an element can have more 8 electrons in nth i.e., outermost shell . This is why elements such as sodium and magnesium have more than 8 electrons in their outermost shells.
The Third Shell and the 18 Electron Capacity
The third period contains only eight elements even though the electron capacity of the third shell is 18. This is because when the other shells get filled and the resultant number of electrons becomes eighteen, it gets added up and settles in the third electron shell and three shells are acquired by the fourth period.
The octet rule is a useful tool for understanding the stability of atoms and their reactivity with other elements. It’s important to remember that the octet rule is just an approximation, and that there are exceptions to it that still lead to stable electron configurations.
Atoms are most stable when their outermost electron shell is full, and this is known as the octet rule. The first shell is filled with 2 electrons, the second is filled with 8 electrons, and the third is filled with 8. Elements such as sodium and magnesium have more than 8 electrons in their outermost shells due to the addition of d and f electrons in the (n−1) and (n−2) shells. The third period contains only eight elements even though the electron capacity of the third shell is 18 because when the other shells get filled and the resultant number of electrons becomes eighteen, it gets added up and settles in the third electron shell.
What is the 8 valence electron rule?
The octet rule is a fundamental concept in chemistry that states that atoms prefer to have eight electrons in their valence shell. This is because these electrons are the outermost layer of electrons in an atom, and having eight of them provides a more stable configuration than having fewer. The eight valence electrons rule is especially important for the main group elements, which are the elements not found in the transition metal or inner-transition metal blocks.
The octet rule was first formulated by Richard Abegg in 1904, and is often referred to as Abegg’s rule. Abegg’s rule states that the difference between the maximum positive and negative valences of an element is often eight. This means that atoms tend to form compounds so that they have eight valence electrons in their outermost shell.
When considering the octet rule, we only take into account the s and p electrons. These are the electrons found in the outermost shells of the atoms, and having eight of them provides a more stable configuration. An octet in these atoms corresponds to an electron configuration ending with s2p6.
Why is the 8 valence electron rule important?
The 8 valence electron rule is important as it helps to explain why certain atoms form certain compounds with other atoms. The rule states that atoms have a tendency to form compounds so that they have eight electrons in their valence shell. This helps to explain why some atoms form certain compounds and why others form different compounds.
The 8 valence electron rule also helps us to understand why some atoms are more reactive than others. This is because atoms that have fewer than eight electrons in their valence shell tend to be more reactive. This is because they are more likely to gain electrons and form more stable compounds.
Exceptions to the 8 valence electron rule
While the 8 valence electron rule is a useful tool for predicting the behavior of atoms, there are some exceptions. For example, some atoms, such as hydrogen, can form compounds with fewer than eight electrons in their valence shell. This is because hydrogen has a tendency to form covalent bonds with other atoms, which can lead to fewer than eight electrons in the valence shell.
Additionally, some atoms, such as those in the transition metal and inner-transition metal blocks, can have more than eight electrons in their valence shell. This is because some of these atoms can have d or f electrons in their outermost shells.
The 8 valence electron rule is an important concept in chemistry that helps us to understand why atoms form certain compounds. It states that atoms prefer to have eight electrons in their valence shell, and when they have fewer than eight electrons they tend to be more reactive and form more stable compounds. While there are some exceptions to this rule, it is still a useful tool for predicting the behavior of atoms and understanding why certain compounds form.



Leave a Comment