Why Does Electrolysis of Water Break Bonds Rather than Boiling It?
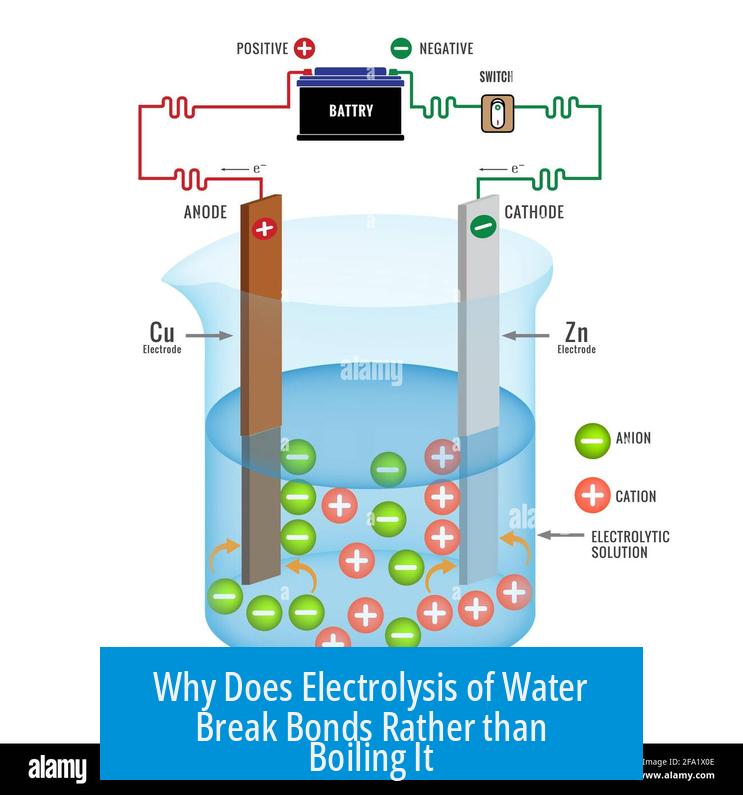
Electrolysis of water breaks molecular bonds because it uses electrical energy to induce chemical changes that split water molecules into hydrogen and oxygen gases. Boiling, on the other hand, is a physical change driven by thermal energy that converts liquid water into vapor without breaking chemical bonds.
1. Difference Between Boiling and Electrolysis
1.1 Boiling as a Physical Change
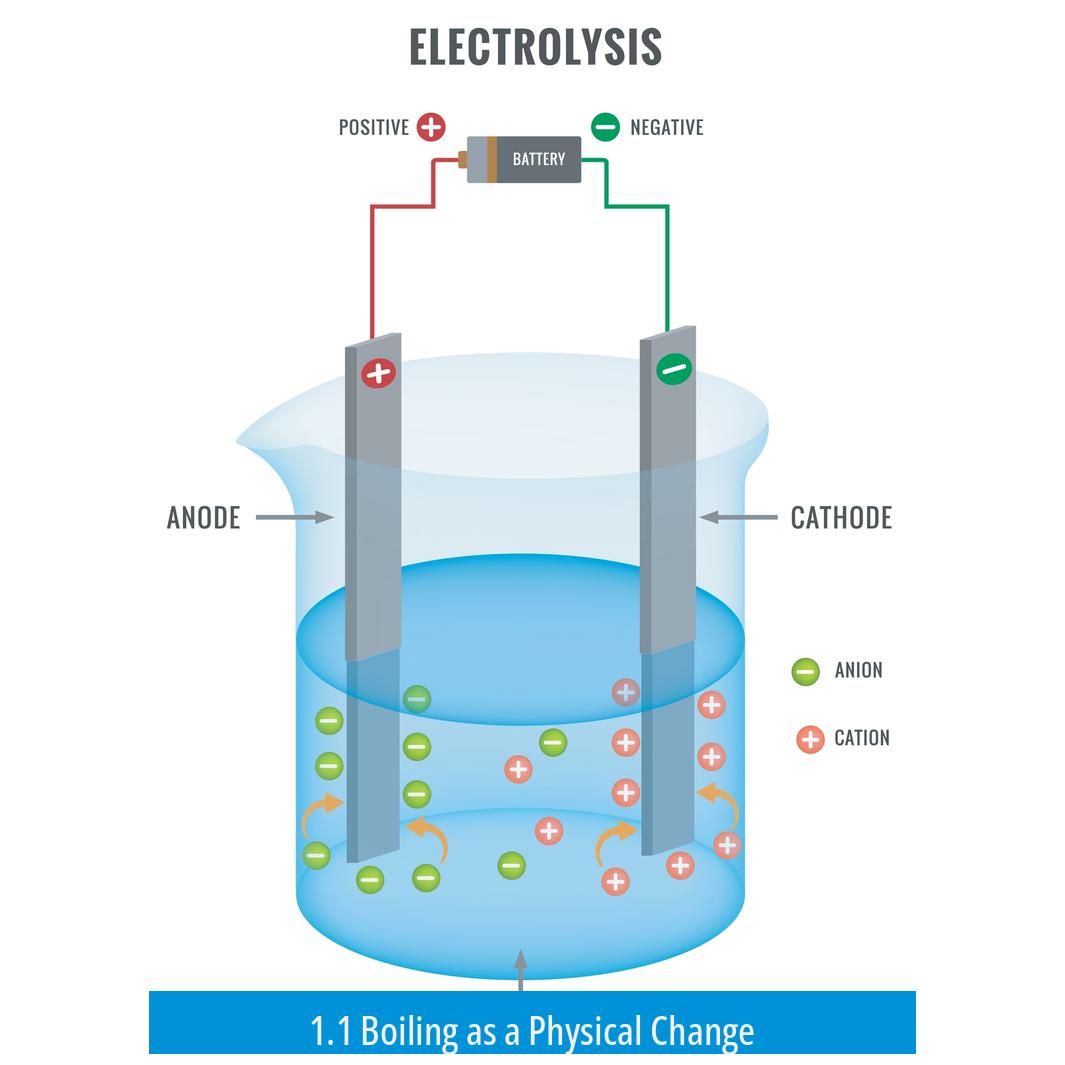
Boiling water involves heating the liquid until molecules gain enough kinetic energy to transition into the gas phase. This change is physical, as water molecules remain intact; they move faster and eventually escape as steam.
Heat directly increases molecular vibrations and motion. When the molecules overcome intermolecular forces, water boils but does not chemically alter.
1.2 Electrolysis as a Chemical Change
During electrolysis, electrical energy causes electrons to transfer at electrodes, triggering a chemical reaction that changes the connectivity of atoms within the molecules.
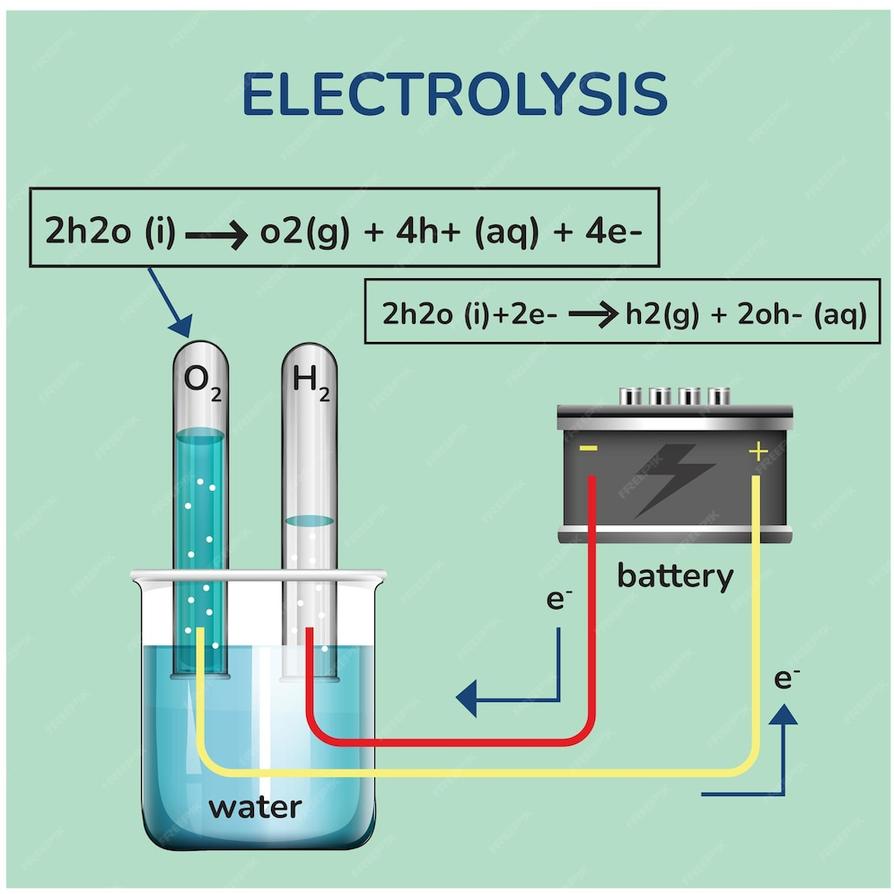
Unlike heat, the energy supplied by electrons can break covalent bonds between hydrogen and oxygen atoms in the water molecule, producing hydrogen gas (H2) and oxygen gas (O2).
2. Nature of Energy Imparted in Electrolysis versus Boiling
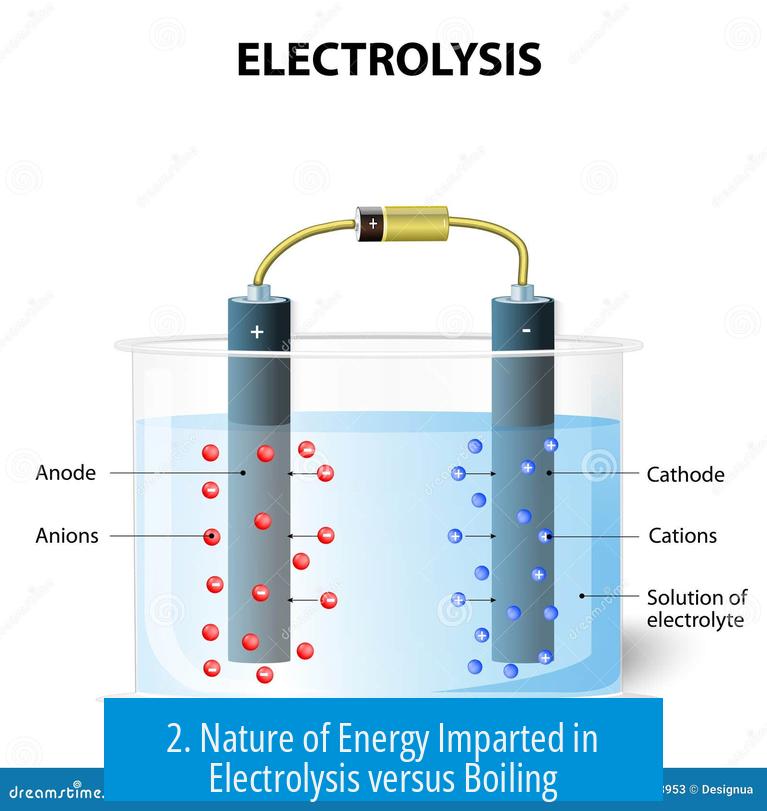
2.1 Heat vs Electrical Energy
Boiling relies on thermal energy, which increases temperature uniformly, causing physical transformation from liquid to gas without bond cleavage.
Electrolysis applies electrical energy through electron flow. This energy targets electrons in water molecules, enabling redox reactions.
2.2 Role of Electrons in Electrolysis
- Electrons added or removed from water molecules cause reduction and oxidation reactions.
- The transfer of electrons destabilizes water molecules, enabling bond breaking.
- This process generates separate oxygen and hydrogen molecules rather than vaporizing water.
3. Physical Environment during Electrolysis
3.1 Electrode Temperature
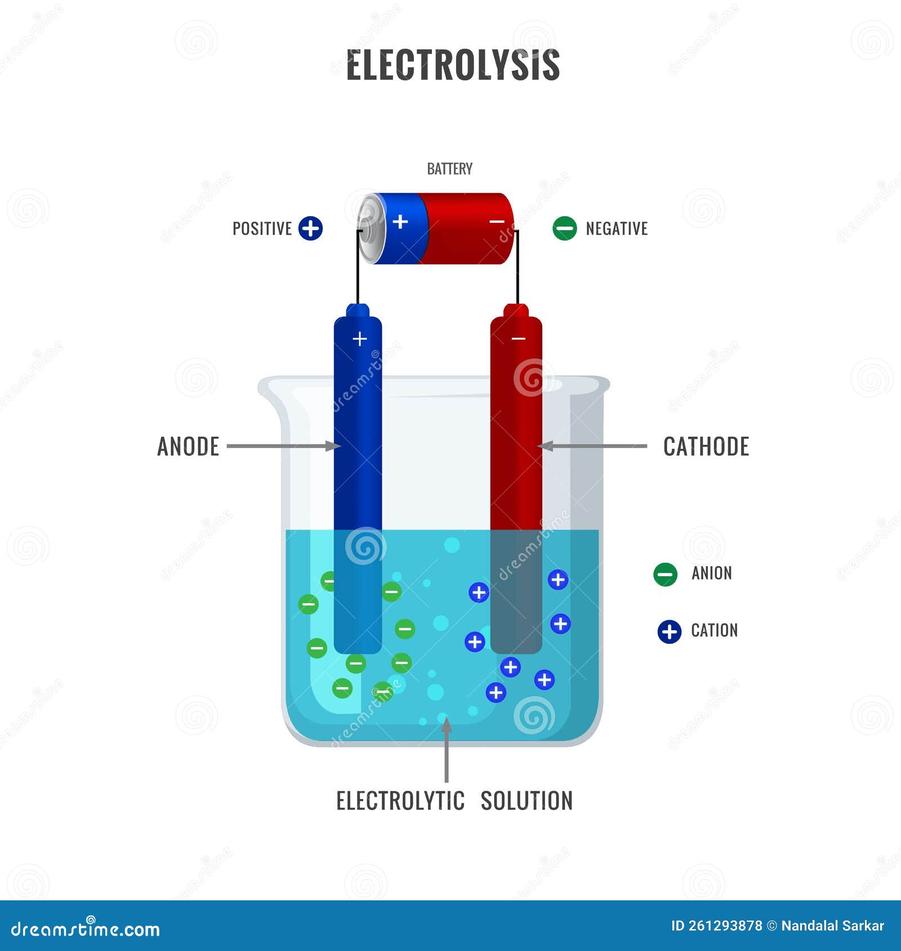
Electrolysis typically occurs at or near room temperature. The electrodes—usually inert platinum rods—do not heat water substantially.
Because heat is minimal, water does not boil during electrolysis. Energy input focuses on electron transfer rather than increasing kinetic energy of molecules.
4. Mechanism of Molecular Breakdown During Electrolysis
4.1 Role of Electrodes and Electric Field
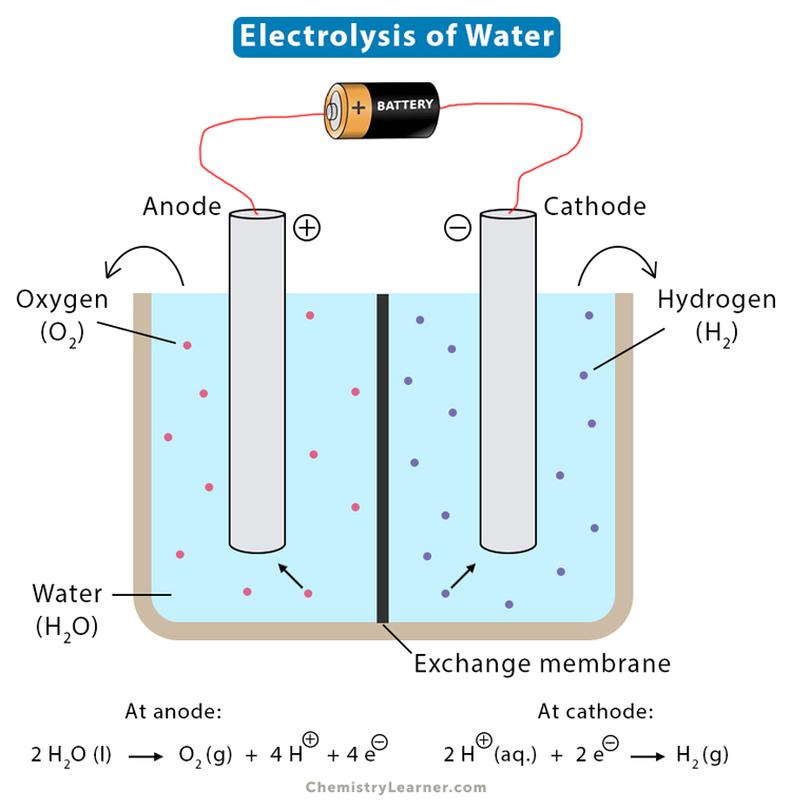
Two electrodes submerge in water: an anode (positive) and a cathode (negative). The electric field created pulls apart water molecules.
At the anode, oxidation occurs (loss of electrons). At the cathode, reduction occurs (gain of electrons).
4.2 Polarity of Water Molecule
Water is polar; oxygen carries a partial negative charge while hydrogens carry partial positive charges.
This polarity influences how the electric field interacts with water molecules, facilitating electron transfer reactions at electrode surfaces.
4.3 Redox Process: Electron Transfer at Electrodes
- Electrons enter vacant orbitals of water molecules at the cathode, reducing H2O to hydrogen gas and hydroxide ions.
- Electrons are removed from water at the anode, oxidizing it to oxygen gas and protons.
- These reactions require voltage high enough to overcome the energy barrier of breaking bonds.
4.4 Formation of Reactive Intermediates
Intermediate species such as hydroxyl radicals (·OH) and superoxide ions arise from electron gain or loss during electrolysis.
These reactive intermediates quickly rearrange, promoting the breakdown of water molecules.
4.5 Rearrangement into Stable Products
Following bond cleavage, hydrogen and oxygen atoms recombine into their diatomic molecular forms (H2 and O2). These gases bubble off the electrodes.
5. Contrasting Boiling and Electrolysis Effects on Water Molecules
5.1 Boiling Does Not Break Molecular Bonds
Boiling water affects molecular motion and phase without breaking the H–O bonds. Molecules escape into vapor unchanged chemically.
5.2 Electrolysis Breaks Molecular Bonds
Electrical energy in electrolysis disrupts water’s internal chemical bonds. The process directly cleaves molecules into separate gases.
| Aspect | Boiling | Electrolysis |
|---|---|---|
| Type of Energy | Thermal (heat) | Electrical (electron transfer) |
| Effect on Molecules | Physical phase change | Chemical bond breaking |
| Temperature | Elevated (100°C at 1 atm) | Usually room temperature |
| Products | Water vapor (H2O) | Hydrogen gas (H2) and oxygen gas (O2) |
| Process | Physical conversion | Redox chemical reaction |
Summary of Key Points
- Boiling is a physical change involving heat increasing molecular motion to vaporize water.
- Electrolysis uses electrical energy to induce electron transfers that break water’s covalent bonds.
- Electrodes create an electric field causing oxidation-reduction reactions at their surfaces.
- Water’s polarity helps facilitate electron transfer, assisting molecular breakdown.
- Boiling keeps the H–O bonds intact; electrolysis breaks these bonds forming H2 and O2 gases.
Why does Electrolysis of Water Break Bonds Rather Than Boiling It?
Simply put, electrolysis breaks the molecular bonds of water by transferring electrical energy via electrons that rearrange atoms into new molecules, while boiling only adds heat energy causing water to evaporate without breaking any bonds. If you’ve ever wondered why zapping water with electricity splits it into hydrogen and oxygen, instead of just making it steam away, here’s the scoop.
At first glance, heating water until it turns to steam and electrolysis might seem similar—they both involve energy and water. But the key difference is the type of energy at play. Boiling involves applying heat, while electrolysis channels electrical energy directly into the water molecules via electrodes.
Boiling: A Physical Change, Nothing More
Boiling water simply heats up the molecules. The heat makes the water molecules jiggle faster and faster, and eventually, they have enough energy to escape the liquid state and become vapor. This transition is a physical change—not chemical. The water molecules stay intact throughout the process.
This means when you boil water, you’re not messing with the molecular bonds between hydrogen and oxygen. You’re just giving the whole molecule enough oomph to move into the gas phase. The H2O molecules stay connected. So boiling water, despite the steam rising, doesn’t affect the molecular structure.
Electrolysis: The Chemical Wizardry of Bond Breaking
Electrolysis, on the other hand, is a chemical change. Rather than heating up the water, it injects electrons at the cathode and pulls electrons away at the anode. This electron shuffle causes redox (reduction-oxidation) reactions that break the bonds holding water molecules together.
Inside the electrolysis setup, two rods of inert metal like platinum dive into the water. One rod acts as the cathode, the other as the anode. These electrodes create an electric field that tugs at water molecules. Instead of making the molecules vibrate faster, these electrodes pull them apart chemically.
The Magic of Electrons: How Bonds Break in Electrolysis
Electrons are the real MVP here. At the cathode, excess electrons are pushed into water molecules, filling empty orbits. This extra electron causes water to reduce, gaining electrons to form hydrogen gas. Meanwhile, at the anode, electrons get plucked away, oxidizing the water molecules to form oxygen gas.
This electron transfer is essential. Without it, water molecules would stubbornly hold onto their hydrogen and oxygen atoms. But with enough voltage pushing electrons across the cell, bonds break and new molecules emerge—H2 and O2 gas.
Polar Nature of Water Aids the Process
Water’s molecular structure also helps. It’s a polar-covalent molecule—a little tug of war between positively charged hydrogens and a negatively charged oxygen. This charge difference makes water responsive to the electric field created by the electrodes, letting the electrons rearrange the atoms more easily.
Reactive Intermediates and Stable Products
During electrolysis, briefly formed reactive intermediates like hydroxyl radicals and superoxide pop up. These radicals are unstable and quickly recombine or react to form the stable gases we know—hydrogen and oxygen. So it’s a messy molecular dance behind the scenes, but the end result is neat: two pure gases that we can collect.
Why Electrodes Stay Cool and No Boiling Occurs
Interestingly, the electrodes remain near room temperature during electrolysis. There’s no significant heat generated to boil the water. Instead, energy input is purely electrical, focused on breaking bonds rather than shaking molecules into gas form.
Summary: Two Roads, One Molecule
| Process | Type of Energy Supplied | Effect on Water | Result |
|---|---|---|---|
| Boiling | Thermal energy (heat) | Increases molecular motion; no bond breakage | Water changes from liquid to gas (steam) |
| Electrolysis | Electrical energy (electron transfer) | Breaks chemical bonds through redox reactions | Water decomposes into hydrogen and oxygen gases |
Here’s a practical question to chew on: If you wanted to split water molecules for fuel, why would you choose electrolysis over boiling? Of course, because boiling won’t break the H-O bonds. It’s clever chemistry, not just some heat frenzy.
Electrolysis unlocks water’s potential by using electrons like tiny molecular locksmiths picking the locks that hold atoms together. Boiling? It just boils the goodies away.
Final Thoughts
Understanding the core difference between these two processes deepens your grasp of energy and molecular science. Electrolysis’s electron-based energy changes chemical connectivity. Boiling only agitates molecules enough to jump into the gas phase without changing composition.
So next time you see steam rising from a pot, remember: those water molecules are still water. But when an electric current hums through an electrolysis cell, bonds break and new substances are born.
That, dear reader, is why electrolysis breaks bonds rather than boiling water.




Leave a Comment