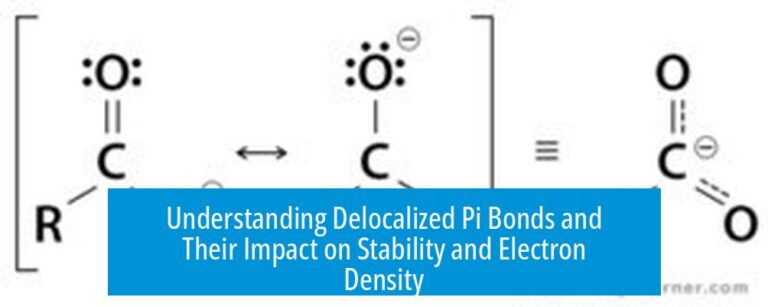
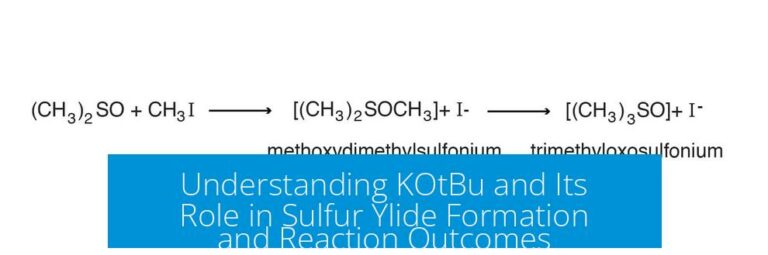
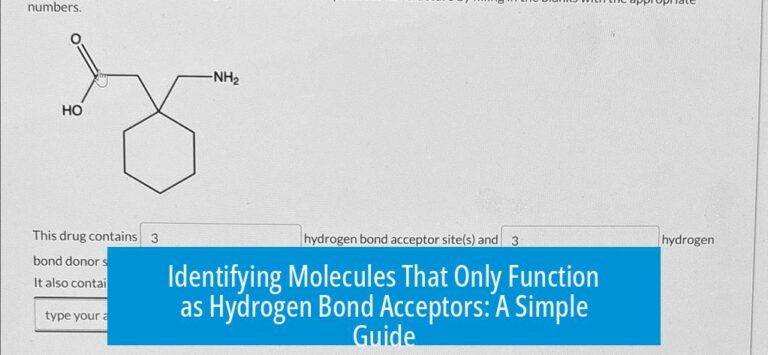
Using ChemDraw and ChemSketch for Effective Chemical Drawings and Tools
Using ChemDraw and ChemSketch for Chemical Drawings ChemDraw offers specialized features like Newman projection templates and an orbitals tool, which streamline chemical structure illustrations. ChemSketch, by comparison, lacks built-in Newman...