Basic Question: What Is the Difference Between a Compound and a Molecule?
A molecule is a specific type of compound defined by atoms held together by covalent bonds, while a compound broadly refers to any stable substance composed of two or more different elements that requires energy to separate its atoms. This distinction lies in the nature of bonding, composition, and structural arrangement of atoms. To understand this difference clearly, it is essential to explore the definitions, bonding types, and examples illustrating both concepts.
1. Definitions and Their Relationship
What Is a Compound?
A compound is a chemical substance that has a definite composition. It always contains atoms of two or more different elements combined in a fixed ratio. This ratio often follows stoichiometric rules, although some compounds deviate slightly in particular cases.
The defining feature of a compound is that it is formed by chemical bonds between different types of atoms, producing a unique substance with distinct chemical and physical properties.
What Is a Molecule?
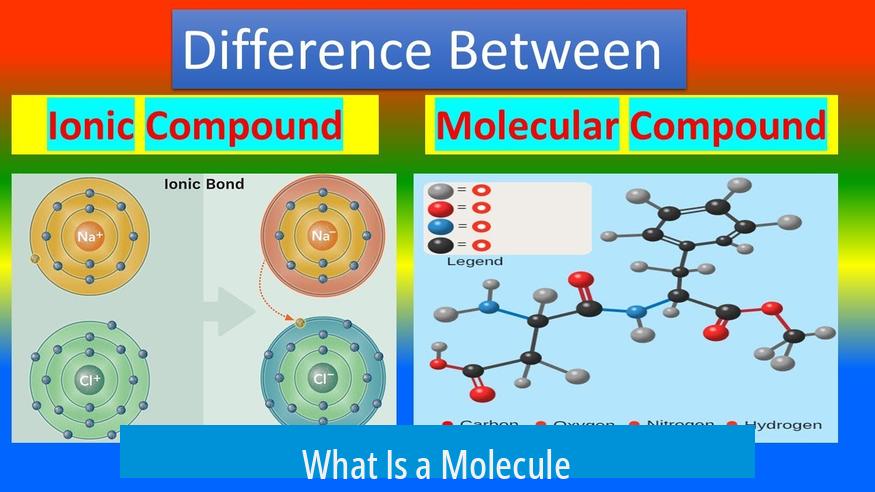
A molecule is the smallest unit of a substance that retains its chemical identity. It consists of two or more atoms bonded together. Molecules can be formed from atoms of the same element or from different elements.
All molecules are compounds only if they contain at least two different elements. For instance, oxygen gas (O2) consists of two oxygen atoms bonded together. It is a molecule but not a compound because it contains only one element.
Relationship Between Compounds and Molecules
- A molecule is a sub-type of compound when it contains two or more different elements.
- Compounds encompass both molecular substances (with covalent bonds) and other forms like ionic compounds.
2. Energy and Bonding Nature
Energy Considerations
Both compounds and molecules involve atoms bonded together in arrangements that require energy input to separate. The bonds represent attractive forces holding the atoms in place, demanding energy to break them apart.
Covalent Bonds in Molecules
Molecules primarily involve covalent bonding, a bond type where atoms share electrons creating an electron density between nuclei. This shared electron density pulls the nuclei together, stabilizing the molecule.
This covalent bonding ensures the atoms remain associated as discrete, identifiable molecular units. Energy is necessary to overcome this attraction and separate the atoms, maintaining the molecule’s integrity.
Other Compound Types and Bonding
Compounds also include other bonding forms such as ionic bonds, metallic bonds, and coordinate covalent bonds.
- Ionic compounds consist of positively and negatively charged ions held together by electrostatic forces.
- These compounds often do not exist as discrete molecules but as extended crystal lattices.
- Energy is still required to overcome ionic attractions, but the structural arrangement differs.
3. Covalent Molecules Versus Ionic Compounds
Molecules as Covalent Compounds
Typically, molecules are formed through covalent bonding and can be found as discrete units with a fixed number of atoms. For example, water (H2O) is a molecule with one oxygen atom covalently bonded to two hydrogen atoms.
In water, each molecule maintains its identity whether in gas, liquid, or solid phases. Even when dissolved, water molecules stay largely intact.
Example: Water vs Table Salt
| Feature | Water (H2O) | Table Salt (NaCl) |
|---|---|---|
| Constituent Elements | Hydrogen and Oxygen (different elements) | Sodium and Chloride (different elements) |
| Bond Type | Covalent bonds within molecules | Ionic bonds within crystal lattice |
| Existence as Discrete Units | Yes, discrete H2O molecules | No, extended lattice structure, no discrete NaCl molecules |
| Structural Arrangement | Individual molecules held by hydrogen bonds | Crystal structure with each Na+ surrounded by six Cl− ions and vice versa |
| Behavior in Solution | Water molecules remain intact | Ions fully dissociate into Na+ and Cl− ions |
Table salt (sodium chloride) forms a crystal lattice where each sodium ion is surrounded by chloride ions and vice versa. There are no distinct NaCl molecules. When dissolved, the crystal dissociates into ions.
This contrasts with water, which retains its molecular units in all states and when dissolved in solvents such as alcohols or acetone.
4. Composition Differences: Elements Involved
Compounds must have two or more different elements chemically bonded. This is a fundamental characteristic. Single-element molecules like O2 or N2 are molecules but not compounds because they contain only one element.
Some molecules contain multiple atoms of the same element, so they fall outside the category of compounds but remain molecules.
Hence:
- Compounds: Two or more different elements chemically combined.
- Molecules: Two or more atoms bonded, possibly the same or different elements.
Summary of Key Differences
- Molecules are specific arrangements of atoms via covalent bonds forming discrete entities, sometimes formed from the same element (O2, N2).
- Compounds are chemical substances composed of two or more different elements bound by chemical bonds, which may be covalent, ionic, or other types.
- Molecules are a subset of compounds when they contain multiple different elements.
- Ionic compounds such as salt are compounds but do not form distinct molecules; rather, they form extended networks.
- Energy input is necessary to break both molecules and compounds apart, but the nature of bonding defines structural and chemical behavior.




Leave a Comment