Understanding the Uses of Empirical Formulas
Empirical formulas represent the simplest whole-number ratio of elements in a compound and are primarily used to determine the elemental composition of substances and to assist in identifying molecule types. They do not indicate the exact number of atoms or molecular structure but offer a foundational understanding in chemical analysis.
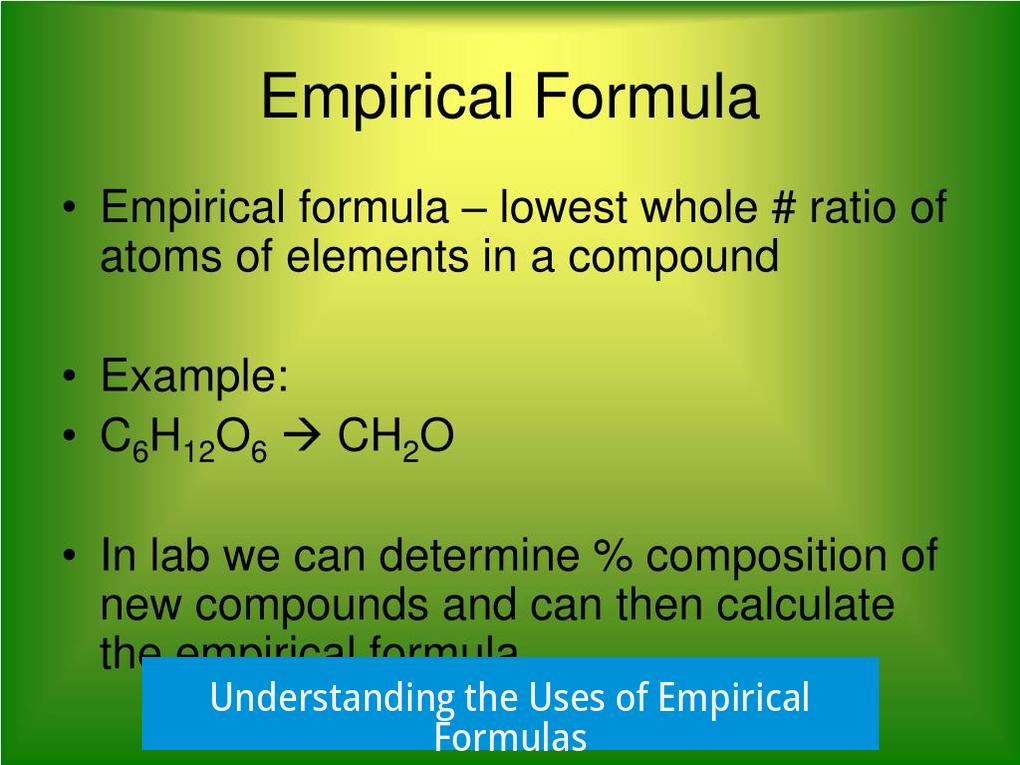
Basic Concept of Empirical Formulas
The empirical formula expresses the simplest ratio of atoms in a chemical compound. It gives the basic proportion of elements but does not provide the molecular formula or the arrangement of atoms. For example, the empirical formula CH2 indicates one carbon atom for every two hydrogen atoms but lacks information about the total number of atoms or bonding.
Role in Elemental Analysis
Empirical formulas are often derived from elemental analysis techniques such as combustion analysis. When an unknown organic compound is burned in oxygen, the amounts of CO2 and H2O produced help determine the proportion of carbon and hydrogen, respectively. This information leads to the simplest ratio of elements present.
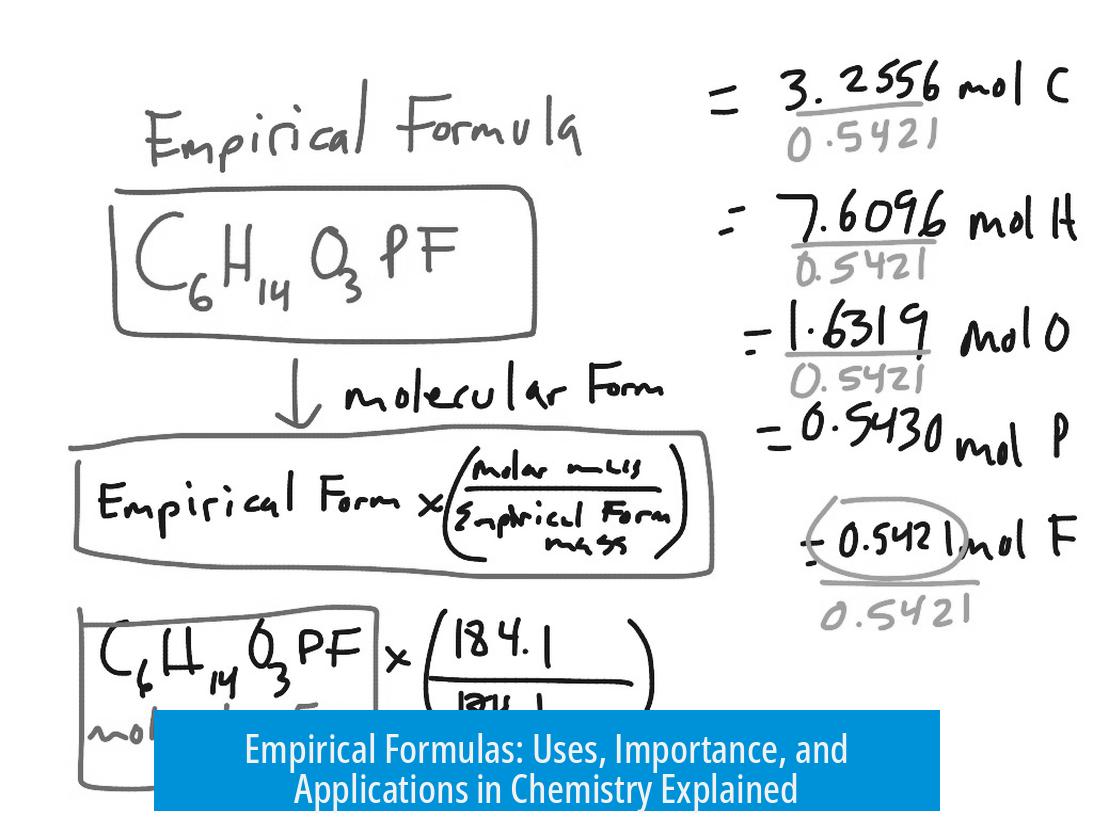
Relation to Molecular Formulas

The empirical formula can be a stepping stone to finding the molecular formula. By dividing the compound’s molecular weight by the empirical formula mass, chemists find the multiplier for the empirical formula. For instance, if a compound’s empirical formula is CH2 and its molecular weight is 112 g/mol, then the molecular formula is C8H16 (since 112/14 = 8).
Hypothesizing Molecular Composition
Chemists use empirical formulas to hypothesize possible molecular formulas. In some scenarios, an empirical formula may suggest ratios that do not correspond to known molecules, which guides scientists to consider multiples or related ratios. For example, an empirical formula C4H4 with a 1:1 ratio may imply the actual molecule is C2H2, a known compound.
Applications in Identifying Compound Families
Empirical formulas help classify groups of compounds sharing similar atomic ratios. For example, all cyclic alkanes and mono-unsaturated aliphatics share the empirical formula CH2, though their molecular formulas differ (general formula CnH2n). This aids in pattern recognition within chemical families.
Limitations and Modern Relevance
While empirical formulas were essential historically, modern analytical tools offer precise molecular and structural information. Therefore, empirical formulas are less critical today but still serve as quick references for elemental ratios and preliminary analysis.
Simplicity Versus Structural Detail
Empirical formulas provide a simplified view of a compound’s composition without addressing its three-dimensional structure or bonding. This makes them useful for quick comparisons and initial assessments but insufficient for detailed molecular understanding.
Key Takeaways
- Empirical formulas show the simplest ratio of elements in a compound.
- They arise from elemental analysis like combustion experiments.
- Molecular formulas are multiples of empirical formulas, calculated using molecular weight.
- Empirical formulas help hypothesize and classify molecular structures.
- Their practical use is limited by modern advanced analytical techniques.
- They offer a quick, simplified representation compared to detailed structural data.
What is the main purpose of an empirical formula?
An empirical formula shows the simplest ratio of elements in a compound. It helps identify elemental composition but does not give the exact number of atoms or molecular structure.
How does the empirical formula relate to the molecular formula?
The molecular formula is a whole number multiple of the empirical formula. By dividing the molecular weight by the empirical formula mass, you can find the exact molecular formula.
Why are empirical formulas important in elemental analysis?
They come from elemental analysis methods, like combustion tests, giving the basic elemental ratios in a sample. This helps in understanding composition before detailed molecular data is available.
Can empirical formulas help guess the actual molecular formula?
Yes. Chemists use the simplest element ratios to hypothesize about the molecular formula or identity, even if the exact formula is unknown at first.
Do empirical formulas provide structural information?
No. They only show elemental ratios. Structural or 3D arrangements require other methods beyond empirical formulas.
Are empirical formulas still useful with modern techniques?
They have limited use now. Modern methods give precise molecular data, making empirical formulas less critical but still useful for quick composition insights.


Leave a Comment