Can We Tell Whether the Bonds in a Molecule Are Polar or Nonpolar Just by Knowing the Dipole Moment of These Molecules?
Knowing only the overall dipole moment of a molecule does not allow one to conclusively determine the polarity of individual bonds within that molecule. Although the dipole moment provides information about the molecular polarity, the complexity of molecular geometry, vector nature of dipoles, and electronegativity differences between atoms make it impossible to rely solely on the dipole moment to assess bond polarity. A full understanding requires analyzing molecular structure, bond vectors, and atomic electronegativities.
Understanding Dipole Moment and Molecular Polarity

The dipole moment is a vector quantity that measures the separation of electric charge within a molecule. It depends on the magnitude and direction of the electronegativity difference between atoms involved in bonds. This property indicates where the electron density is concentrated and is essential for determining molecular polarity.
- The dipole moment arises when atoms in a bond do not share electrons equally.
- The vector nature means dipole moments have both magnitude and direction.
- Overall molecular dipole moment results from vector addition of individual bond dipoles.
Because dipoles are vectors, molecular geometry heavily influences whether dipoles add constructively or cancel out.
Role of Dipole Moment in Molecular Polarity

When all bond dipoles in a molecule point in the same general direction, the molecule exhibits a net dipole moment and is polar. For instance, molecules like water (H2O) show significant net dipole moments due to bent shapes causing dipole vectors to add up.
However, dipole moments can cancel if equal and opposite dipoles exist due to molecular symmetry, leading to a nonpolar molecule despite the presence of polar bonds.
Why Dipole Moment Alone Does Not Reveal Bond Polarity
While the overall dipole moment describes molecular polarity, it does not specify whether individual bonds are polar or nonpolar. This arises because:
- Dipole moment is a cumulative measure of all bond dipoles.
- Vector cancellation can reduce or nullify the net dipole moment.
- Each bond’s polarity depends on differences in electronegativity between its two atoms.
Therefore, a molecule may have polar bonds but a zero net dipole moment due to symmetrical geometry. Conversely, a molecule with a nonzero dipole moment must have polar bonds, but the overall dipole moment alone cannot disclose which bonds are polar or nonpolar.
Electronegativity and Bond Polarity
Bond polarity fundamentally arises from electronegativity differences between bonded atoms. Atoms with higher electronegativity attract electron density more strongly, creating a partial negative charge at that atom and partial positive charge at the other.
- Greater electronegativity difference → stronger bond polarity.
- Covalent bonds between atoms of similar electronegativity are nonpolar.
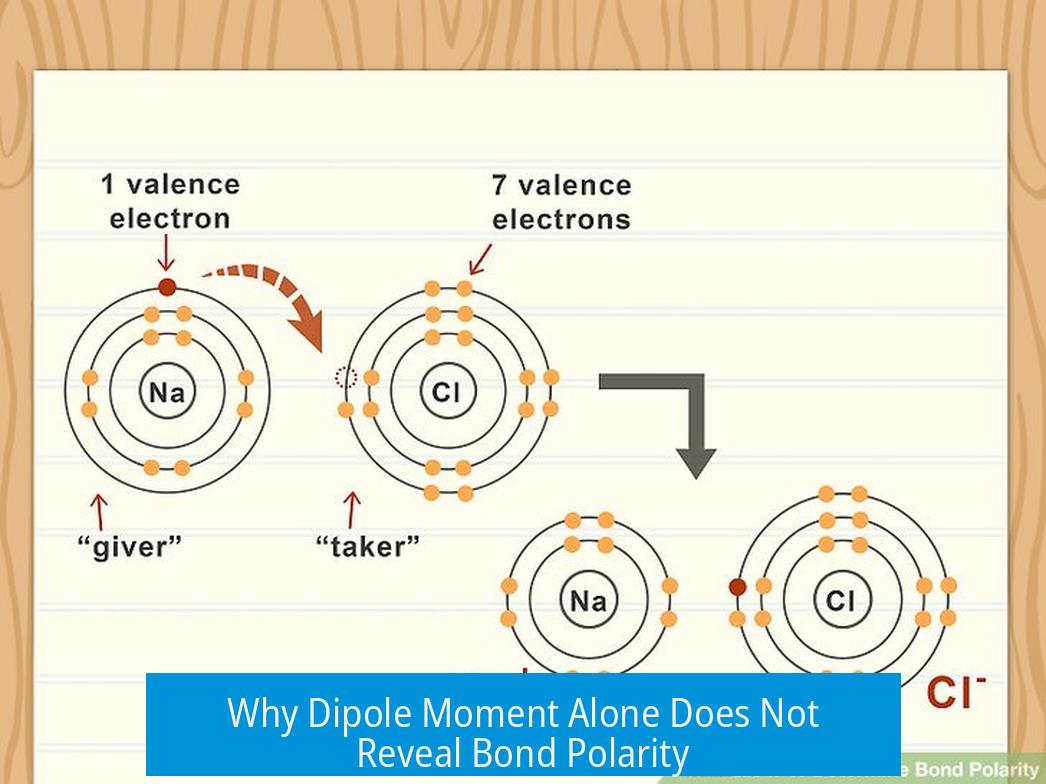
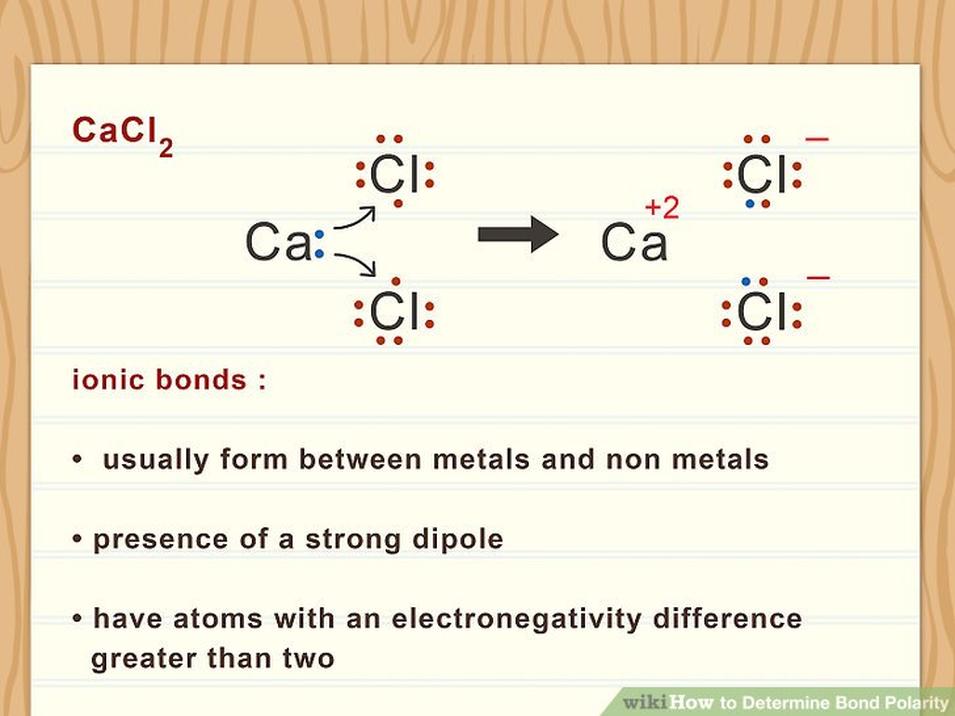
- Ionic bonding involves full electron transfer and is distinct from bond polarity.
Thus, evaluating electronegativity values is necessary to assess bond polarity beyond knowing dipole moments.
Importance of Molecular Geometry
The shape of a molecule critically impacts whether bond dipoles reinforce or negate each other.
- Geometry dictates the spatial orientation of bond dipoles.
- Symmetrical shapes often cause dipoles to cancel completely.
- Asymmetrical shapes allow dipoles to combine into a net dipole moment.
Vector addition of bond dipoles requires understanding geometry via models such as VSEPR (Valence Shell Electron Pair Repulsion). Without this, dipole moment data offers incomplete insight.
Illustrating Geometry’s Effect on Dipole Moments
| Molecule | Geometry | Dipole Moment Findings | Bond Polarity Insights |
|---|---|---|---|
| Carbon Dioxide (CO2) | Linear | Two equal polar bonds point in opposite directions; net dipole moment = 0 | Bonds are polar; molecule is nonpolar due to vector cancellation |
| Ozone (O3) | Bent | Dipole vectors point downward towards outer oxygens; net dipole moment ≠ 0 | Polar bonds cause a polar molecule; geometry prevents cancellation |
| Carbon Tetrachloride (CCl4) | Tetrahedral | Four equal C–Cl dipoles symmetrically arranged; net dipole moment = 0 | Bonds are polar; geometry cancels dipoles, molecule is nonpolar |
Implications of Vector Addition in Dipole Moments
It is critical to view dipole moments as vectors. The overall molecular dipole is their sum, which means:
- Opposing dipoles can neutralize each other.
- Magnitude of individual bond dipoles and their spatial angles determine the net dipole.
This vector property elucidates why molecules with polar bonds may have zero overall dipole moments.
Summary Teaching Analogy on Bond Polarity
Explaining bond types through electron sharing aids in conceptual clarity:
- Nonpolar bonds: equal sharing of electrons by both atoms.
- Polar bonds: unequal sharing, one atom partially “owns” electrons more.
- Ionic bonds: effectively full electron transfer, with no sharing.
This electron-sharing approach emphasizes that polarity depends on atomic interactions, not solely on dipole moments.
Final Considerations
Knowing a molecule’s dipole moment assists in determining if the molecule is polar or nonpolar. However, it cannot reliably specify polarity of individual bonds because:
- Dipole moments represent net molecular polarity after vector addition.
- Geometry influences the resultant dipole orientation.
- Electronegativity differences govern bond polarity independently of net dipole.
To fully understand bond polarity, one must assess:
- The electronegativities of atoms involved in each bond.
- The molecular geometry and bond angles.
- The vector nature of dipole moments and their spatial directionality.
Key Takeaways
- Dipole moments describe overall molecular polarity but not individual bond polarity.
- Bond polarity depends on electronegativity differences between atoms in the bond.
- Molecular geometry determines how individual bond dipoles combine or cancel.
- Molecules can have polar bonds yet exhibit zero net dipole due to symmetry.
- Complete analysis for bond polarity requires knowledge of electronegativity and molecular structure along with dipole moment.
Can we tell whether the bonds in a molecule are polar or nonpolar just by knowing the dipole moment of these molecules?
Simply put: No, knowing only the dipole moment of a molecule does not reliably tell you whether its individual bonds are polar or nonpolar. This may sound like a quick “no,” but there’s a fascinating story woven into the science behind dipole moments, molecular geometry, electronegativity, and bond polarity.
Let’s unpack this puzzle together.
The Dipole Moment: Your Molecule’s Directional GPS
The dipole moment is a vector quantity—it has both magnitude and direction. It reflects the overall separation of charge in a molecule, showing you where the electron density tends to hang out.
Imagine you’re looking at molecules like tiny tug-of-war games with electrons. The dipole moment points toward the atom pulling the electrons closer (the more electronegative atom). If you see a dipole moment, you know there is some imbalance in electron distribution. But that’s just the headline. The details matter.
For example, in molecules like ozone (O3), the geometry bends the molecule in such a way that the dipole moment doesn’t cancel out. So, it shows a net dipole moment, indicating overall polarity. That’s handy to know.
Why Geometry Plays the Referee in This Electron Drama
Now, here’s the kicker. Even if each bond in a molecule is polar, the SHAPE of the molecule can cause these dipole moments to cancel out when added as vectors.
Take carbon dioxide (CO2): it has two polar double bonds between carbon and oxygen. Oxygen, being more electronegative, pulls electron density toward itself. So, each bond has a dipole. But because CO2 is linear, these two dipoles point in exactly opposite directions and cancel each other out perfectly. Result? The entire molecule has zero net dipole moment and is overall nonpolar.
Similarly, carbon tetrachloride (CCl4) has four polar C–Cl bonds. Chlorine’s electronegativity pulls electrons toward itself, creating bond dipoles. But the molecule’s tetrahedral symmetry spreads these dipoles evenly in space. The vector sum is zero, leading to no net dipole moment. So CCl4 is nonpolar even though its bonds are polar.
So, does a net dipole moment of zero mean all bonds are nonpolar? Nope.
Electronegativity: The Real Player Behind Bond Polarity
Polarity at a bond level depends on electronegativity differences between the atoms involved. This is independent of the molecule’s overall dipole moment.
- If atoms equally share electrons, the bond is nonpolar.
- If one atom pulls electrons more strongly, the bond is polar.
For instance, in CCl4, carbon and chlorine differ enough in electronegativity to create polar bonds. You won’t tell this just by looking at the molecule’s dipole moment, which is zero.
Here’s a memorable teaching trick for those learning: nonpolar bonds are like parents sharing 50-50 custody of gifted electrons. Polar bonds are when one parent gets the kids most of the time, and the other has weekend visits. Ionic bonds are basically “sole custody.” This analogy emphasizes that bond polarity is about electron distribution between two atoms, not the entire molecule.
The Limitations of Using Dipole Moment Alone
Dipole moment works well as a clue for overall molecular polarity, but it cannot disclose the hidden secrets of every individual bond inside. Polar bonds might hide behind molecular shapes where dipole moments cancel out. Nonpolar bonds, on the other hand, won’t contribute to the dipole moment at all.
So, relying solely on dipole moment without considering molecular geometry and electronegativity differences can mislead you.
Putting It All Together: What Really Determines Bond Polarity?
- Check the electronegativity difference between two bonded atoms. If it’s significant, the bond is polar.
- Examine the molecular geometry using VSEPR theory. The shape dictates how individual bond dipoles add up.
- Consider the vector nature of dipole moments. Opposite vectors can cancel, resulting in net zero dipole moment despite polar bonds.
Think of vectors like directions on a compass. Two arrows of equal length point north and south? They sum to nothing.
Real-Life Example Recap
| Molecule | Bond Polarity | Dipole Moment | Geometry | Notes |
|---|---|---|---|---|
| CO2 | Polar bonds (C=O) | Zero (vectors cancel) | Linear | Polar bonds but nonpolar molecule |
| O3 | Polar bonds | Nonzero | Bent | Molecular bends cause net dipole |
| CCl4 | Polar bonds (C–Cl) | Zero | Tetrahedral | Symmetrical shape cancels dipole |
How to Truly Determine Bond Polarity?
Measure or look up electronegativities of atoms. Check the molecule’s shape. Then, consider dipole moments of each bond—not just the total molecular dipole moment. This holistic approach reveals hidden polar bonds even when the molecule seems nonpolar at first glance.
It’s like reading a mystery novel: A single clue (dipole moment) isn’t enough. You want the whole story (electronegativities, geometry, vector addition) to crack the case.
Wrapping Up
In short, dipole moments offer a helpful overview for molecular polarity but don’t provide the full picture of bond polarity. Because bond polarity depends on the interplay of electronegativity and geometry, just knowing the dipole moment isn’t like reading a book’s summary—it’s more like seeing the cover. You get a hint, but not the full plot.
The next time you hear “dipole moment,” remember: It’s a powerful tool—but only when paired with other molecular details. So, don’t judge bonds by their dipole moment alone!
Curious about your favorite molecules? Grab those electronegativity charts and VSEPR models. Dive deeper. You might discover some polar surprises lurking where you least expect.



Leave a Comment