Understanding D2O Consumption: Safety, Biological Effects, and Practical Considerations

D2O consumption, or the intake of heavy water, is generally safe in small amounts but can disrupt biological processes and become toxic at high levels due to isotopic effects. It is non-radioactive, unlike tritium, and its unique chemical properties impact metabolism and cellular functions when consumed excessively.
What Is D2O?
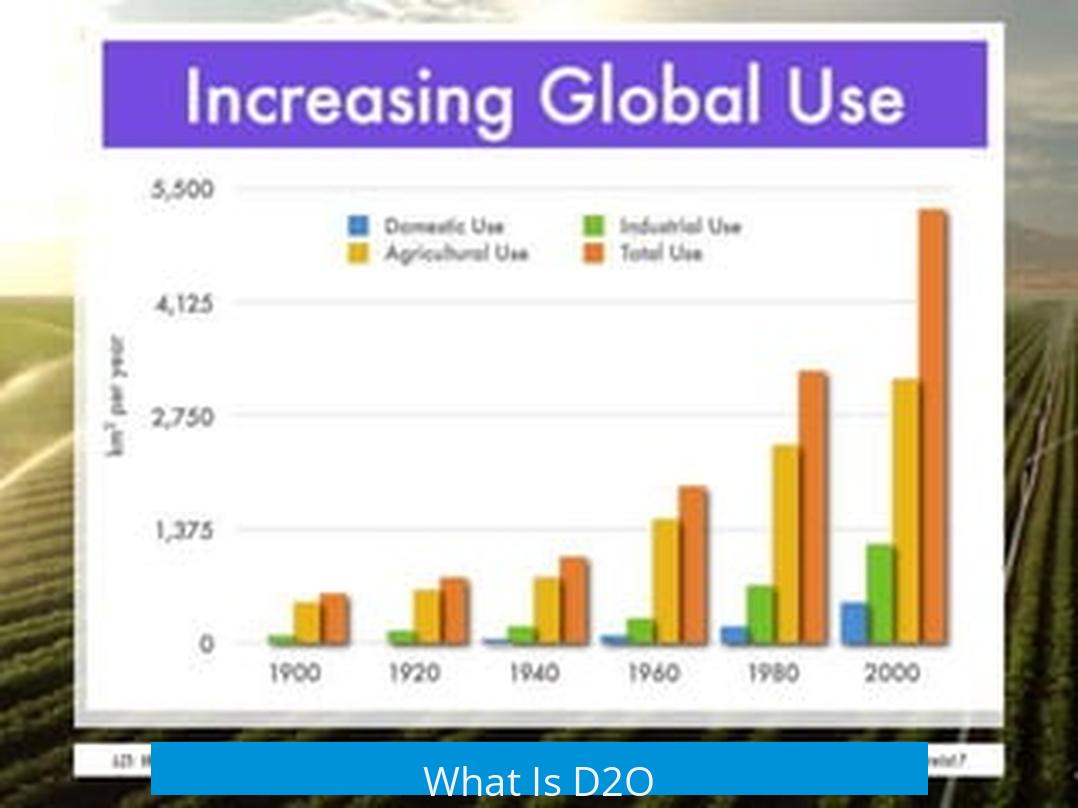
D2O, commonly called heavy water, differs from normal water (H2O) by containing deuterium atoms instead of regular hydrogen. Deuterium is a stable isotope of hydrogen with one neutron, doubling the hydrogen’s mass. This isotopic substitution leads to slightly altered chemical and physical properties, while maintaining a similar structure to ordinary water.
1. Safety and Toxicity of D2O Consumption

D2O is safe in small quantities because it is non-radioactive and chemically similar to water. It naturally exists in trace amounts in ordinary water, typically about 0.015%. Humans and animals consume small amounts without harm.
However, high concentrations pose toxicity risks. Research and experiments indicate lethality begins at around 30% of body water replaced by D2O. This is a substantial volume—much higher than concentrations encountered naturally or from casual consumption.
- Small sips or even a glass of D2O are unlikely to cause harm.
- Consuming a liter might cause mild effects but is unlikely to be fatal.
- Animal studies (notably Soviet-era deuterated dog experiments) observed animals remain healthy up to 50% D2O content.
- Illness and mortality occur when D2O comprises about 70–75% of body water.
- Death results from metabolic disruption triggered by isotope effects on enzyme kinetics.
The toxicity arises from the substitution of hydrogen with deuterium, which changes chemical reaction rates essential to metabolism.
2. Biological Effects and Mechanisms of D2O Consumption

Deuterium’s increased mass causes slower chemical reaction rates. This phenomenon, known as the kinetic isotope effect, affects enzymatic reactions involving water molecules.
Many metabolic processes rely on rapid hydrolysis and other water-mediated steps. When water atoms are replaced with D2O, these reactions slow down. Enzymes using water as a substrate or product perform less efficiently, which can disturb cellular metabolism.
Key biological effects include:
- Slowed enzymatic reactions: Processes like hydrolysis or energy metabolism often require water; replacing it with D2O delays these steps.
- Disruption of cell division: Cells actively dividing incorporate D2O into nucleic acids and proteins, slowing replication and growth.
- Potential long-term risks: Animal studies suggest high D2O intake might increase cancer risk and infertility, presumably due to interference with DNA synthesis and repair.
Short-term consumption of small amounts is unlikely to cause significant metabolic misregulation, but sustained or heavy use could be harmful.
3. Radioactivity Status of D2O

D2O is not radioactive. Deuterium is a stable isotope of hydrogen and does not emit ionizing radiation. This property distinguishes heavy water from tritiated water, which contains the radioactive isotope tritium (3H).
- Deuterium isotope: stable and non-radioactive.
- Tritium isotope: weak beta emitter, radioactive, and toxic in ingestion.
Confusion sometimes arises between these isotopes, but natural heavy water poses no radiation risk. The presence of deuterium in natural water is normal and safe.
4. Practical Use and Observations Related to D2O Consumption

D2O features unique chemical and physical properties valuable in scientific research and practical demonstrations.
For example:
- Microbiologists grow certain microbes in media comprised entirely of heavy water, demonstrating that some life forms tolerate pure D2O quite well.
- D2O ice cubes sink in regular water due to their higher density, which can be applied in educational contexts or novelty drinks.
- Caution is necessary regarding contaminants since industrially produced D2O may contain residual impurities not related to the isotope itself.
While these uses show relative biological tolerance, they represent specialized cases. Routine consumption is uncommon and generally unnecessary.
Summary of Key Points on D2O Consumption
- D2O is chemically like water but contains the stable isotope deuterium, making it heavier.
- Small amounts of D2O do not cause harm; it naturally occurs in drinking water.
- Substantial intake (around 30% of body water replaced) disrupts metabolism and can be lethal.
- Enzymatic reactions slow down due to isotope effects, affecting cell division and metabolic balance.
- Heavy water is non-radioactive and distinct from tritiated water, which is radioactive and harmful.
- Biological tolerance to D2O varies, but pure D2O media can support some microbial life.
- Commercial sources may contain impurities, so caution is advised when handling D2O.
Additional Considerations
Anyone investigating or using D2O should work with it understanding both its chemical nature and biological impacts. While experiments with animals and microbes inform safe exposure levels, human effects require careful control due to potential metabolic disruptions.
In scientific fields such as biochemistry and molecular biology, D2O serves as a useful tracer and solvent. Its unique properties enable analysis of metabolic pathways and hydrogen bonding behavior.
Overall, responsible management of D2O respects its similarities to normal water while acknowledging its significant kinetic isotope effects at higher concentrations.
The Truth About d2O Consumption: Safety, Effects, and Science Behind Heavy Water
Is drinking d2O (heavy water) safe? Yes, but only in small amounts. Heavy water, chemically known as deuterium oxide, differs from regular water by having hydrogen atoms replaced with their heavier sibling, deuterium. But don’t freak out — this swap isn’t radioactive, and sipping a bit won’t do any harm.
So what exactly happens if you gulp down a large quantity? Picture this: Soviet scientists experimented with dogs drinking heavy water, dubbing one the “deuterated dog.” Up to around 50% heavy water content in the body, the dog was just fine — healthy and normal. But as this climbed towards 70-75%, grim consequences followed, culminating in death. In fact, to reach lethal levels in humans, you’d need D2O amounting to roughly 30% of your body weight, way more than ethanol required to produce fatal intoxication. So in everyday life, encountering heavy water is quite harmless.
What about radioactivity? Can heavy water give you radiation sickness?
Nope. D2O is not radioactive. This is a common misconception, often confusing deuterium with tritium, another hydrogen isotope that is radioactive. Deuterium is stable, so the heavy water you might drink or handle emits no radiation at all. This contrasts with tritiated water (3H2O) used as a tracer in labs, which you should avoid ingesting. Natural water always contains small amounts of heavy water — roughly 0.015% — part of the water we drink daily without concern.
How does heavy water affect your body’s chemistry?
This is where things get interesting (and a bit tricky). Heavy water isn’t a perfect substitute for regular water when it comes to your metabolism. The difference in mass between hydrogen and deuterium causes what’s called the kinetic isotope effect. Simply put, enzymatic reactions involving water slow down when deuterium takes hydrogen’s place.
Many vital biological processes depend on water. Hydrolysis reactions (breaking molecular bonds with water) can take longer to complete in heavy water. Cell division is particularly sensitive — the hydrogen atoms play crucial roles in molecular machinery that replicates DNA and builds new cells.
Long-term or high consumption of D2O might lead to metabolic misregulation. Studies link such conditions to increased cancer risk and infertility, probably because the slowed reactions disrupt normal cell growth and repair. But remember, these risks only emerge with substantial heavy water ingestion.
Can microbes survive in heavy water?
Surprisingly, yes! Microbes can grow in media made with 100% heavy water. This indicates a degree of biological tolerance and adaptability that’s impressive. It also means that D2O can replace H2O in certain lab cultures, highlighting just how chemically similar they are, despite the hefty isotope swap. Evolution must love a challenge.
What are some fun or practical applications of D2O?
Heavy water’s unique physical properties open neat doors. For instance, ice cubes made from D2O actually sink when placed in ordinary water, because D2O ice is denser. Imagine impressing your friends at a party with this quirky cocktail trick—”Look, my ice cubes defy physics!” Just watch out for any contaminants in commercially produced D2O, as impurities might pose more risk than the isotope itself.
So, should you experiment with drinking heavy water?
Small sips or an occasional glass probably won’t hurt you. But guzzling liters? Probably unwise. Most people won’t encounter dangerous amounts naturally, and there’s no benefit to heavy water over regular water for hydration. It’s more of a scientific curiosity than a super-drink.
Here are some practical tips if you’re curious: never substitute D2O for your daily water intake. If you want to explore the isotope’s effects, observe it in controlled lab settings—through microbial growth or physical experiments with ice density, as an example. And always source your D2O from reputable suppliers to avoid nasty impurities.
In Conclusion
d2O consumption is safe in small amounts but can cause serious metabolic disturbances when taken in large quantities. This is due to the kinetic isotope effect, which slows metabolic reactions involving water. While it’s non-radioactive and occasionally found in nature, heavy water differs enough chemically to disrupt enzymatic functions at elevated levels. The “deuterated dog” experiments vividly show these biological limits.
Curious minds might enjoy the fascinating properties of heavy water—from microbial growth to density-defying ice cubes—but stick to regular H2O for health and safety. Still, isn’t it amazing how a tiny change—just swapping out hydrogen for deuterium—can create such a dramatic biological and physical ripple?
Is drinking small amounts of D₂O dangerous?
Small amounts of D₂O, like a sip or even a glass, are generally safe. It does not produce radiation and naturally occurs in water. Toxic effects only happen at much higher levels.
What happens if someone consumes large amounts of D₂O?
Consuming about 30% of body mass in D₂O can be lethal. High concentrations disrupt metabolism due to kinetic isotope effects, slowing down essential enzymatic reactions and causing sickness or death.
Does D₂O consumption affect cell division?
Yes, D₂O interferes with hydrogen properties in cells, especially dividing cells. This disruption can slow cell processes and may increase risks like infertility and cancer with prolonged high intake.
Is D₂O radioactive or does it emit radiation?
D₂O is not radioactive. It contains deuterium, a stable hydrogen isotope. Confusion often comes from tritium, a radioactive hydrogen isotope, but D₂O itself emits no radiation.
Can microbes grow in pure D₂O?
Most microbes can grow using media made with 100% D₂O, showing some biological tolerance. However, growth rates and metabolism may change due to isotope effects.





Leave a Comment