How to Make Sodium Hydroxide at Home or with Common Materials
Making sodium hydroxide (NaOH) at home or from common materials is technically possible but highly impractical and dangerous. Instead, buying commercially available NaOH, such as solid drain cleaners or hardware store lye, is safer, easier, and more cost-effective.
Why Making Sodium Hydroxide at Home Is Not Recommended
Sodium hydroxide is an essential chemical used in many applications. However, homemade production poses significant hazards and technical challenges. Here are key reasons to avoid making it yourself:
- Production can release toxic chlorine gas which is harmful to health.
- Handling NaOH requires strict safety measures to prevent severe burns.
- The process demands specialized equipment, such as electrolytic cells.
- Purchasing is inexpensive and widely accessible.
Experts strongly recommend buying sodium hydroxide rather than attempting homemade synthesis due to these risks and difficulties.
Commercial Sources of Sodium Hydroxide
Instead of making NaOH, the best approach is to buy it from commercial sources:
- Drain openers: Solid drain cleaners contain nearly pure sodium hydroxide. They come in small packs or large tubes.
- Hardware stores: Products like “Red Devil Lye” often contain NaOH suitable for various uses.
- Bulk suppliers: Larger quantities can be purchased from chemical suppliers or online stores at reasonable prices.
Choosing cheaper, additive-free products is preferable to avoid impurities such as perfumes or surfactants.
Safety Precautions When Handling Sodium Hydroxide
Sodium hydroxide is highly corrosive and dangerous. Proper safety precautions are essential:
- Always wear gloves, eye protection, and a lab coat when handling NaOH.
- Avoid direct skin contact because NaOH can cause chemical burns by reacting with skin oils and tissues.
- If you feel a soapy or slippery sensation on your skin after contact, wash immediately with water to prevent damage.
- Never mix NaOH with incompatible substances, especially acids or strong oxidizers.
Handling NaOH without necessary precautions can lead to serious injury or long-term health effects.
Homemade Methods to Make Sodium Hydroxide
Some methods exist theoretically and in practice for producing sodium hydroxide from common materials. These procedures range from simple chemical reactions to electrolytic techniques but generally involve significant challenges and hazards.
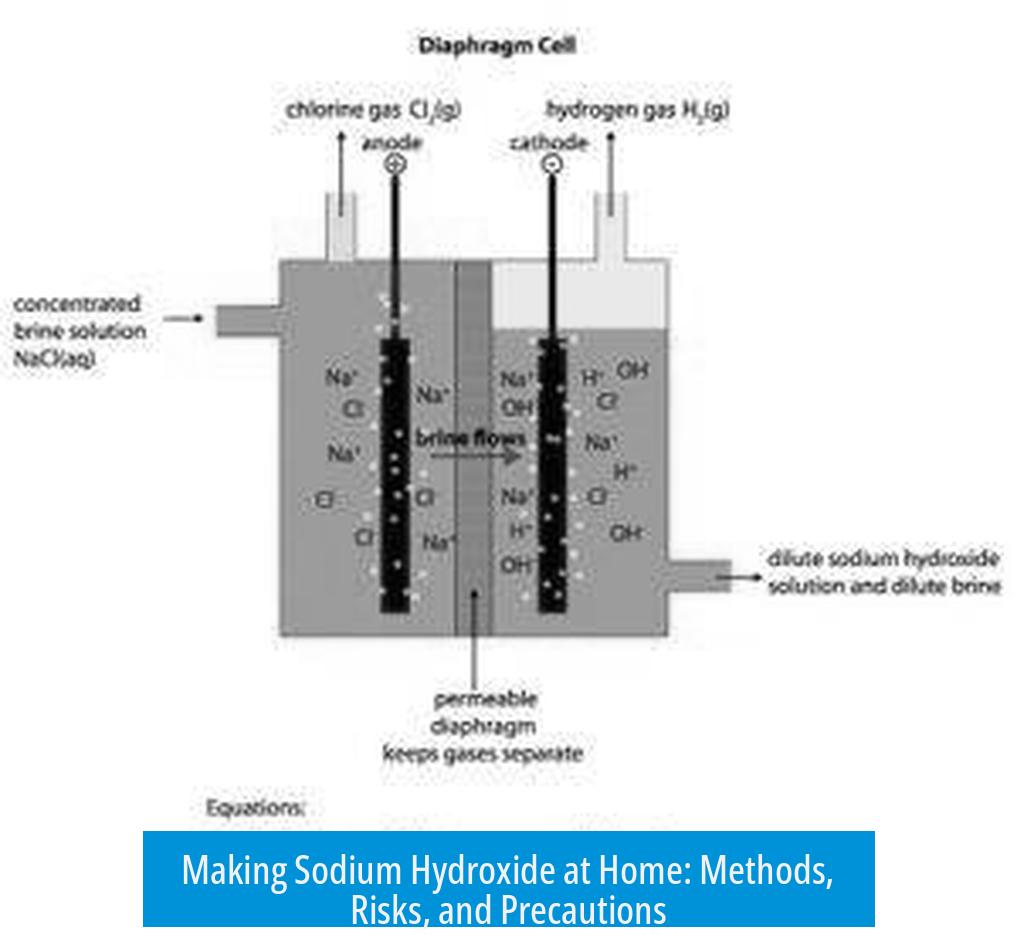
Electrolysis of Sodium Chloride (Salt) Solution
Electrolysis of brine (salt dissolved in water) produces sodium hydroxide, hydrogen gas, and chlorine gas:
| Cathode reaction | Anode reaction |
|---|---|
| 2H2O + 2e- → H2 + 2OH- | 2Cl- → Cl2 + 2e- |
This requires a suitable electrolytic cell, electrodes, and power supply. Managing the chlorine gas generated is a critical safety concern since it is toxic and corrosive.
Professional setups sometimes use mercury or specialized membranes to isolate sodium hydroxide. Home setups lack these controls, increasing the danger of exposure and contamination.
Reaction of Calcium Hydroxide with Sodium Carbonate
A common reaction in basic chemistry involves mixing calcium hydroxide (slaked lime) with sodium carbonate to form sodium hydroxide and precipitate calcium carbonate:
Ca(OH)2 + Na2CO3 → 2 NaOH + CaCO3(s)
This reaction produces NaOH in aqueous form but requires access to pure starting compounds. The sodium hydroxide solution then needs to be isolated and concentrated, which is difficult without laboratory equipment. Industrially, this method is rarely employed for pure NaOH production due to complexity.
Traditional or Folk Methods: Ash and Rainwater
Historically, lye was sometimes made by leaching rainwater through wood ashes. The ashes contain potassium and some sodium carbonate, which dissolve in water to yield alkaline solutions.
Boiling this solution concentrates the alkali, roughly approximating lye. However, the resulting product is impure, variable, and mainly contains potassium hydroxide rather than sodium hydroxide.
While interesting, this method lacks precision and safety for modern applications.
Highly Dangerous Methods: Sodium Metal Reacting with Water
Metallic sodium reacts vigorously with water to produce sodium hydroxide and hydrogen gas:
2Na + 2H2O → 2NaOH + H2
This reaction is extremely exothermic and can cause explosions or fires. Obtaining sodium metal is difficult and costly, and handling sodium metal requires advanced expertise and equipment.
This method is not suitable for non-professionals and should be avoided.
Adding Sodium to Water and Evaporating
One theoretical method involves dissolving sodium in water to produce NaOH, then evaporating the water to isolate solid NaOH. However, this simply restates the reactive process above and shares the same risks.
This approach is neither practical nor safe without professional control.
Summary of Key Points
- Homemade production of sodium hydroxide is unsafe and complex. It involves toxic chlorine gas and dangerous reactions.
- Purchasing is the best option. Solid drain openers or hardware store lye provide affordable, nearly pure NaOH.
- Safe handling of NaOH is critical. Always use gloves, goggles, and protective clothing to avoid burns.
- Some chemical methods exist for lab-scale production, but require specialized equipment and chemicals.
- Traditional ash leaching produces impure alkali mostly potassium-based, and is not a true source of sodium hydroxide.
Can I make sodium hydroxide at home using table salt and water?
Yes, by performing electrolysis on a saltwater solution, you can produce sodium hydroxide. However, this releases dangerous gases like chlorine and hydrogen, making the process risky without proper equipment and ventilation.
Is it safe to make sodium hydroxide by reacting calcium hydroxide with sodium carbonate?
This chemical reaction can produce sodium hydroxide and calcium carbonate. It’s possible but requires measuring and balancing chemicals carefully. Safety gear is necessary as the products and reagents can be hazardous.
Can I get sodium hydroxide from common store products?
Yes. Many drain openers or products labeled as “Red Devil Lye” contain nearly pure sodium hydroxide. Buying these is cheaper and safer than trying to make it yourself at home.
What are the risks of making sodium hydroxide at home?
The main risks include exposure to toxic gases, chemical burns, and explosions. Homemade methods often create chlorine gas or require handling reactive metals, both of which pose serious dangers.
Is the traditional method of filtering rainwater through ashes effective for making sodium hydroxide?
This old method can produce a weak alkaline solution but is unreliable and impure. It cannot replace commercial sodium hydroxide products and requires boiling to concentrate.




Leave a Comment