How Acidic Would a Wedge of Lemon Really Make Water?
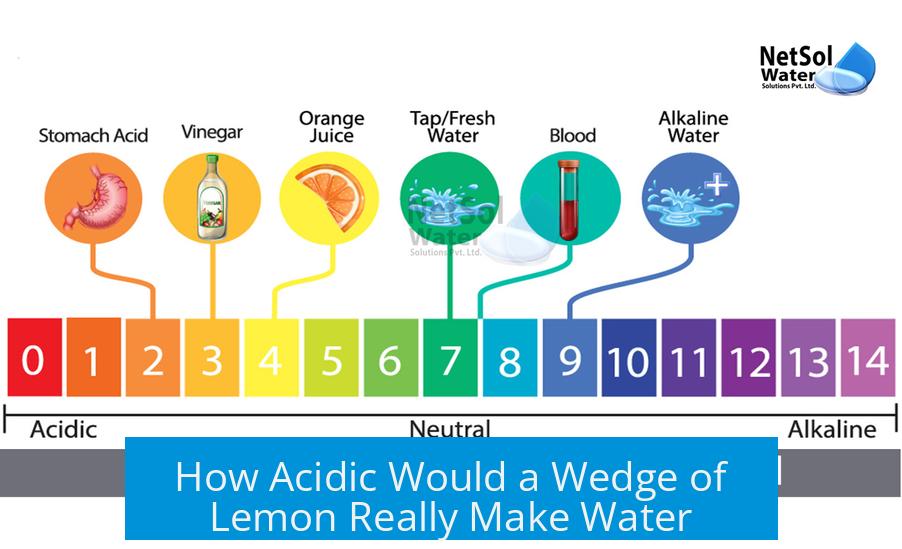
A wedge of lemon generally lowers the pH of water to about 5 or 6, making it mildly acidic but nowhere near as acidic as pure lemon juice or soda.
pH Comparison: Lemon Juice vs. Water
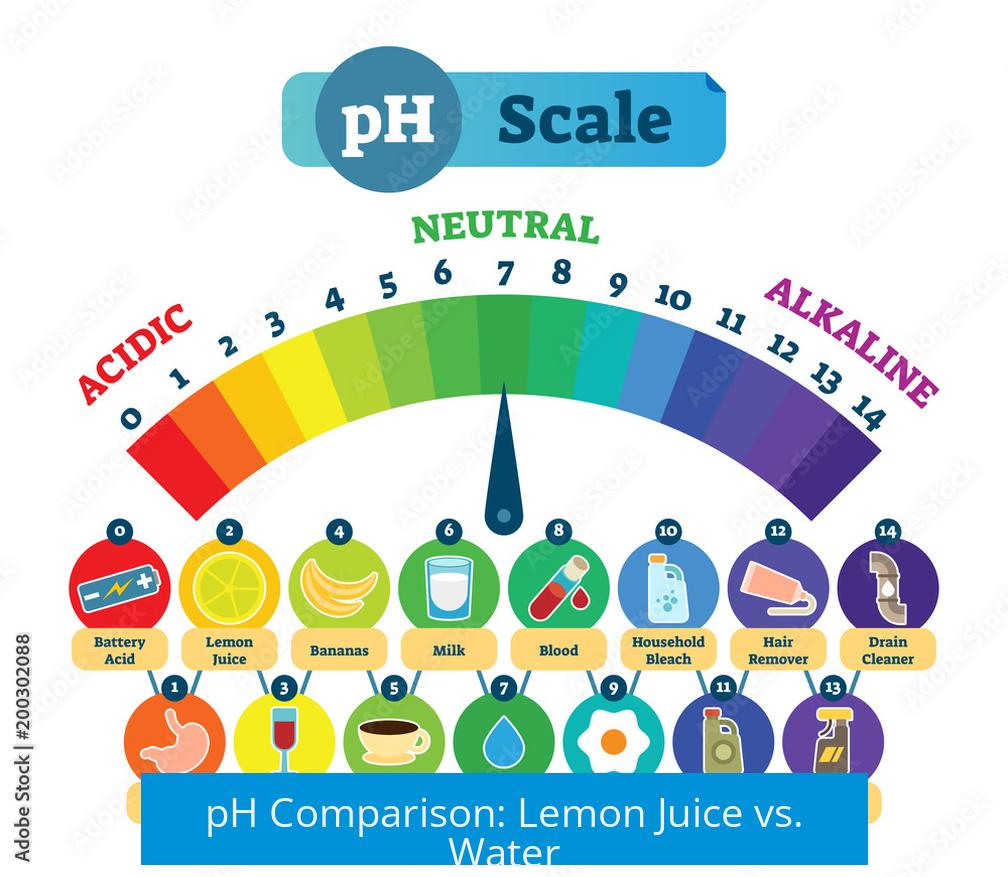
Lemon juice has a pH around 3 due to its citric acid content. In contrast, tap water usually sits close to neutral, around pH 7. Because pH is logarithmic, even small additions of acid can shift pH noticeably.
Effect of Adding Lemon Juice to Water
- A wedge of lemon adds far less juice than a teaspoon — which itself is only about 1.3% lemon juice in 12 oz water.
- This small acid addition lowers the pH from neutral to roughly between 5 and 6.
- Not all the acid in the wedge dissolves into the water, so the actual pH drop is small.
Quantitative Estimation of Citric Acid Impact
One lemon has about 50 mL of juice, containing roughly 5% (2.5 g) citric acid. If all this acid were fully released into 100 mL water, dissociating completely, the pH could drop to about 0.4—very acidic.
However, these assumptions do not hold true in practice since citric acid is a weak acid and does not fully dissociate.
Realistic pH of Lemon-Infused Water
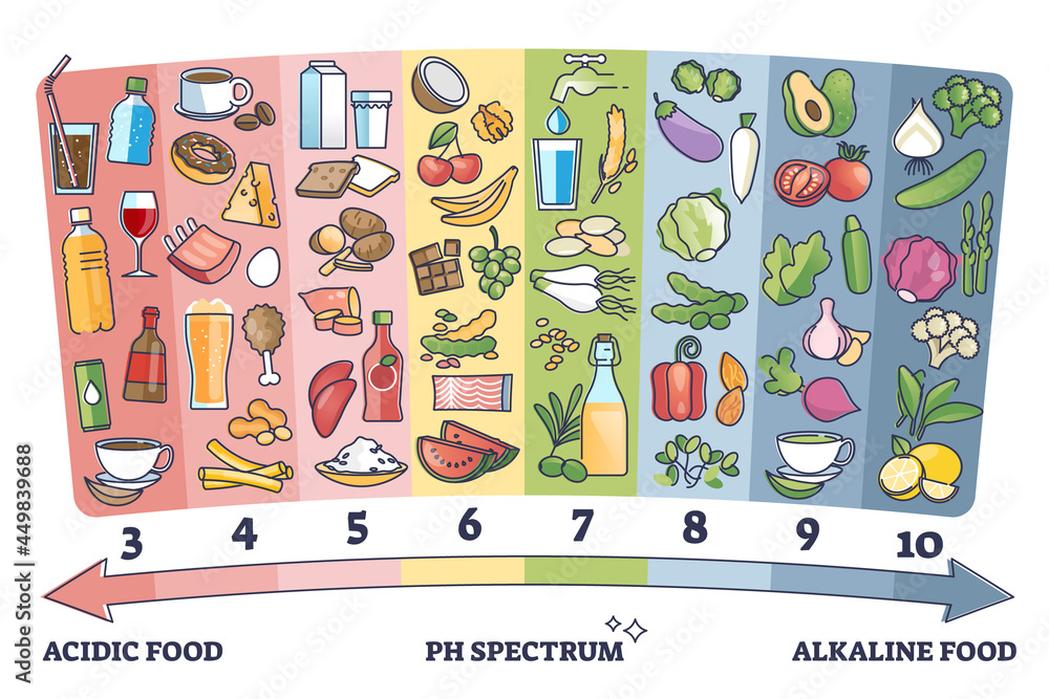
- Typical 1% citric acid solutions exhibit a pH around 2.3, similar to soda.
- A lemon wedge delivers much less acid than 1%, so the pH of lemon water is significantly higher.
- Practical measurements and estimates indicate a pH of approximately 5 to 6 for water with a wedge of lemon.
Implications for Tooth Enamel
The mild acidity of lemon water (pH 5-6) has minimal effects on tooth enamel compared to sodas with pH values near 3. Enamel erosion from acidity occurs mainly with prolonged exposure to stronger acids.
Key Takeaways
- Lemon juice is acidic (~pH 3), but adding a wedge to water only mildly lowers the pH.
- A wedge of lemon lowers water pH to around 5 or 6, depending on dilution and acid release.
- Citrate is a weak acid and does not fully dissociate, limiting acidity.
- Lemon water acidity is far less than soda or pure lemon juice.
- Impact on tooth enamel from lemon water is minimal under typical consumption.
How Acidic Would a Wedge of Lemon Really Make Water?
If you chuck a wedge of lemon into your glass of water, just how acidic does that water actually become? Is it a tiny, barely noticeable shift? Or does it suddenly turn into a citric acid punch rivaling your favorite soda? Let’s find out.
First, some basics: pure tap water usually sits comfortably at around pH 7 — that’s neutral. Lemon juice clocks in at about pH 3, thanks to its citric acid content. Now, because pH is a logarithmic measure, a change from 7 to 3 is massive. But here’s the catch: tossing just a wedge of lemon into your water doesn’t dump all that acid in there.
pH, Citric Acid, and the Lemon Wedge Reality
Lemon juice is roughly 5% citric acid. A whole lemon holds about 50 milliliters of juice — which translates to approximately 2.5 grams of citric acid or around 13 millimoles. If you were to pour the whole lemon into 100 milliliters of water and assumed total dissociation of the acid, you’d end up with a pH near 0.4. That’s very acidic — think stomach acid level.
But here’s where reality throws a curveball: citric acid doesn’t fully give up its protons in solution like a strong acid would. It’s a weak acid, so our pH estimate overshoots the mark.
What Happens When You Add Just a Wedge?
Most people add a lemon wedge to a whole glass of water — say 12 ounces (about 355 milliliters). A wedge contains far less juice than a full lemon, and not all of that juice blends into the water; some stays locked in the pulp. The effective acid concentration drops significantly.
For instance, a teaspoon of lemon juice (about 1/6 ounce) in 12 ounces of water is just around 1.3% concentration. That’s a small amount of acid diluting the water. This typically nudges the water’s pH from a neutral 7 down to roughly 5.5 or 6.
So realistically, the water becomes mildly acidic but nowhere near the tooth-etching level of your everyday soda (which usually sits around pH 2.5 to 3.5).
Putting Numbers Into Perspective
| Liquid | Typical pH |
|---|---|
| Tap Water | ~7 (Neutral) |
| Lemon Juice | ~3 |
| Lemon Water (wedge in glass) | ~5.5 to 6 |
| Coca Cola | ~2.5 |
See? Lemon water’s pH isn’t crazy acidic. In fact, it’s closer to mildly acidic apple juice than stomach acid.
Will That Sour Water Hurt Your Teeth?
Here’s a common worry: could sipping lemon water damage your teeth? Enamel erosion is a real threat with acidic drinks, but remember—lemon water’s acidity is modest. Soda, much more acidic and loaded with sugar, is the real villain causing tooth enamel erosion over time.
With lemon water’s pH hovering near 5.5 or 6, it’s unlikely to cause significant enamel damage unless you swig it all day long and never rinse your mouth. Drinking through a straw or rinsing with plain water after lemon water can help minimize any risk.
Why Does This Matter? Benefits and Myths
Despite the mild acidity, many rave about lemon water’s health perks—hydration, vitamin C boost, and aiding digestion. While the acid might suggest a “kick,” the truth is you’re simply sweetening (and souring) your water without turning it into a citrus bomb.
And for those worried about acidity wrecking their hydration, rest assured, lemon water hydrates as well as plain water. Plus, a subtle pH shift isn’t going to throw your body’s tightly regulated pH out of whack.
Final Thoughts — Should You Squeeze or Slice?
If you want more lemon flavor and a bit more acidity, squeezing juice directly will have a stronger effect than mere wedges. Just remember the pH won’t nosedive dramatically with a wedge alone.
So next time you add lemon to water, know this: you get a fresh, slightly tangy splash, but not a chemical acid test. It’s a splash of zest, not a laboratory experiment gone wild.
Fancy yourself as a lemon enthusiast? Try measuring the pH at home using pH strips. It’s a simple way to geek out on your citrus habits and confirm the mild but pleasant acidity!
In summary, a lemon wedge in water nudges pH lower but won’t turn your drink into anything close to strong acid. Just enough zing, without the sting.
How much does a wedge of lemon lower the pH of water?
A wedge of lemon typically lowers the pH of water to around 5 or 6. This is because the amount of citric acid released is small compared to the water volume.
Why doesn’t lemon juice make water very acidic?
Citric acid in lemon juice is not strong enough to fully release H3O+ ions. The acid only partially dissociates, so the pH remains moderately acidic rather than very low.
How does the pH scale work when mixing lemon juice and water?
The pH scale is logarithmic, so even a small addition of acid can change pH noticeably. However, a wedge of lemon adds too little acid to drop pH below 5 significantly.
Could lemon water harm tooth enamel like soda does?
Unlikely. Lemon water’s pH is higher (5 to 6) than soda (2.5 to 3.5), and enamel damage usually requires prolonged exposure to stronger acids.
What is the difference in acidity between lemon juice and a lemon wedge in water?
Pure lemon juice has a pH around 3, but a wedge only releases a fraction of that acid into water, making the final solution less acidic, closer to pH 5 or 6.



Leave a Comment