How Do Grignard Reagents React with Alcohol?
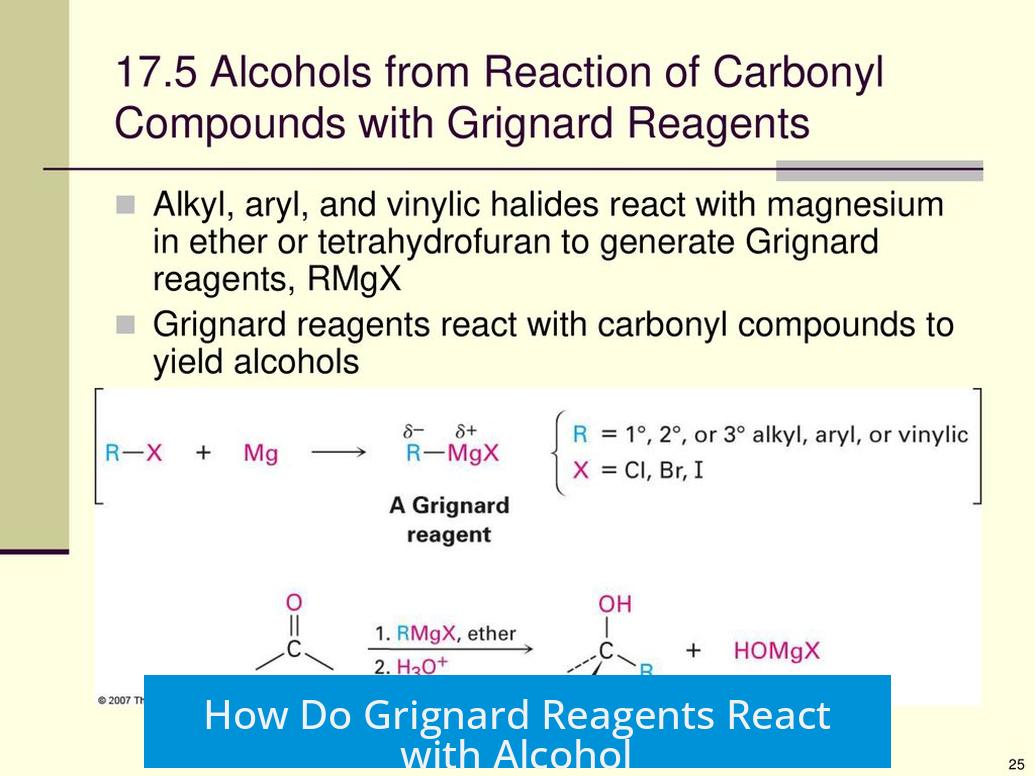
Grignard reagents do not directly add to alcohols but instead act as strong bases that deprotonate the alcohol’s O-H group, resulting in the destruction of the Grignard reagent. This reaction prevents further useful transformations involving the Grignard reagent when alcohols are present.
Grignard Reagents Function as Bases Toward Alcohols

Grignard reagents contain a carbon-magnesium bond, where the carbon behaves as a nucleophilic carbanion equivalent. However, when exposed to alcohols, this nucleophile preferentially acts as a strong base.
- The acidic proton on the alcohol’s hydroxyl (-OH) group is abstracted by the Grignard reagent.
- This proton transfer forms an alkane corresponding to the Grignard’s alkyl group and an alkoxide ion from the alcohol.
This base-induced deprotonation destroys the original Grignard reagent’s reactivity, as the reactive carbanion source is lost.
Acidic Protons Quench Grignard Reagents
Grignard reagents are highly sensitive to compounds with acidic protons. Alcohols fall into this category due to their O-H acidic hydrogen.
- Compounds with lower pKa values than alkanes or alkenes—such as alcohols, amines (N-H), water, carboxylic acids, and alkynes—readily protonate Grignard reagents.
- The resulting protonation neutralizes the nucleophilic carbon and yields alkanes from the electrophilic proton source.
- This protonation prevents Grignard reagents from performing desired carbon-carbon bond-forming reactions.
Interaction with N-H or O-H Containing Compounds
Any molecule possessing N-H or O-H bonds rapidly reacts with Grignard reagents:
- The Grignard reagent deprotonates the acidic hydrogen on N or O.
- The former acidic site becomes negatively charged (alkoxide or amide ion).
- The Grignard’s carbanion is converted into an alkane, thus “consuming” the reagent.
This widespread reactivity with hydrogens on nitrogen or oxygen prevents Grignard reagents from surviving in environments containing alcohols or amines.
Dependence on the Type of Alcohol
The extent of reaction between Grignard reagents and alcohols depends on the specific alcohol structure:
- Simple aliphatic alcohols tend to fully quench Grignard reagents by protonation.
- In molecules where alcohol functionality coexists with other reactive groups such as alkyl halides (R-Br) or carboxylic acids (RCOOH), Grignard reagents may still react selectively with the intended functional group despite the presence of alcohol.
- No validated synthetic method uses alcohols directly to form ethers or other products by reaction with Grignard reagents.
This indicates that while alcohols generally destroy Grignards, some tolerate them under carefully controlled conditions, especially if their acidic protons are less reactive or inaccessible.
Examples of Alcohol Tolerance
Certain alcohol derivatives, especially phenolic hydroxyl groups, can coexist with Grignard reagents without immediate destruction of the latter.
A notable example exists where phenolic OH groups tolerate Grignard reagents, allowing selective reactions elsewhere in the molecule. This tolerance stems from the unique acidity and electronic environment of phenols compared to aliphatic alcohols.
Phenolic Hydroxyl (Phenylic OH) and Grignards
Phenylic or phenolic OH groups exhibit higher resistance to Grignard reagent destruction:
- Resonance stabilization in phenols reduces the ease of proton transfer to the Grignard reagent.
- This allows some phenolic alcohols to persist in the presence of Grignard reagents.
- Such tolerance can be leveraged in synthesis to protect phenolic groups during Grignard reactions targeting other sites.
Summary of Grignard Reagents and Alcohol Interaction
- Grignard reagents act as strong bases toward alcohols, removing the acidic proton from O-H bonds.
- This deprotonation destroys the Grignard, turning it into an alkane and leaving an alkoxide ion.
- Any compound with a proton more acidic than a typical alkane or alkene quenches Grignard reagents by protonation.
- Direct reaction between alcohols and Grignards to form ethers or other coupling products does not occur.
- Some alcohol functionalities, particularly phenolic OHs, can tolerate Grignard reagents under specialized conditions.
- Careful choice of reaction conditions and substrates is critical when Grignard reagents coexist with alcohols.
How do Grignard reagents interact with the O-H bond in alcohols?
Grignard reagents act as bases and remove the acidic proton from the alcohol’s O-H bond. This deprotonation neutralizes the Grignard reagent, preventing it from performing further reactions.
Can Grignard reagents form ethers directly with alcohols?
No, there are no known procedures where Grignard reagents directly react with alcohols to form ethers or similar coupling products. The reaction mainly results in the loss of Grignard activity by proton transfer.
Why do some alcohols tolerate Grignard reagents better than others?
Phenolic OH groups show higher tolerance to Grignard reagents. This is likely due to differences in acidity or resonance stabilization, allowing some phenolic alcohols to coexist with Grignards under certain conditions without destroying the reagent.
What happens if a Grignard reagent contacts any compound with N-H or O-H bonds?
The Grignard reagent deprotonates the N-H or O-H bonds, destroying itself and producing a negatively charged nitrogen or oxygen species. This quenching stops the expected nucleophilic addition reactions of the Grignard.
Can alcohol presence affect Grignard reactions involving other functional groups?
Yes. Alcohols can quench Grignard reagents before they add to other functional groups, but sometimes molecules with alcohol functions are tolerable, depending on the specific reaction conditions and the type of alcohol involved.




Leave a Comment