How Is H3O+ a Strong Acid?
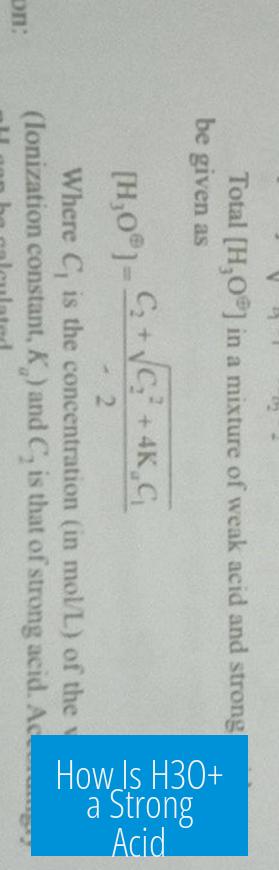
H3O+ is a strong acid due to its readily donating proton, resulting from its nature as the conjugate acid of water, combined with rapid proton transfer dynamics and its position in acid-base equilibria. Although a free proton (H+) does not exist in solution, the hydrated proton, represented as H3O+, exhibits high acidity through fast proton dissociation and transfer, defining the acid strength in aqueous systems.
The True Nature of H3O+ in Water
Protons (H+) do not exist freely in aqueous solution. Instead, they immediately associate with water molecules, forming hydronium ions (H3O+). This ion acts as a better representation of the proton in solution. In fact, the actual proton may be complexed by multiple water molecules, sometimes described as H9O4+ or H15O7+, but H3O+ is the simplest usable model to illustrate the presence of a hydrated proton.
This hydration reflects proton instability in isolation. A free proton rapidly attaches to a water molecule because it cannot maintain stability on its own. Thus, when chemical equations depict free H+, this is a simplification, and the actual species involved is H3O+ or a larger hydrated proton cluster.
Proton Transfer Dynamics and the Grotthuss Mechanism
H3O+ ions do not remain static inside solution. Protons move rapidly between water molecules by a process called the Grotthuss mechanism. Instead of physically moving through the solvent, protons “hop” from one water molecule to another. This hopping makes proton transfer extremely fast, facilitating the acid’s strong behavior.
Because of this dynamic, H3O+ ions are transient. They readily give up protons to neighboring molecules and accept protons back swiftly, creating a continuous equilibrium. This rapid interchange underpins the reactivity and strong acidity observed in solutions containing H3O+.
Defining Acid Strength by Proton Donation Ease
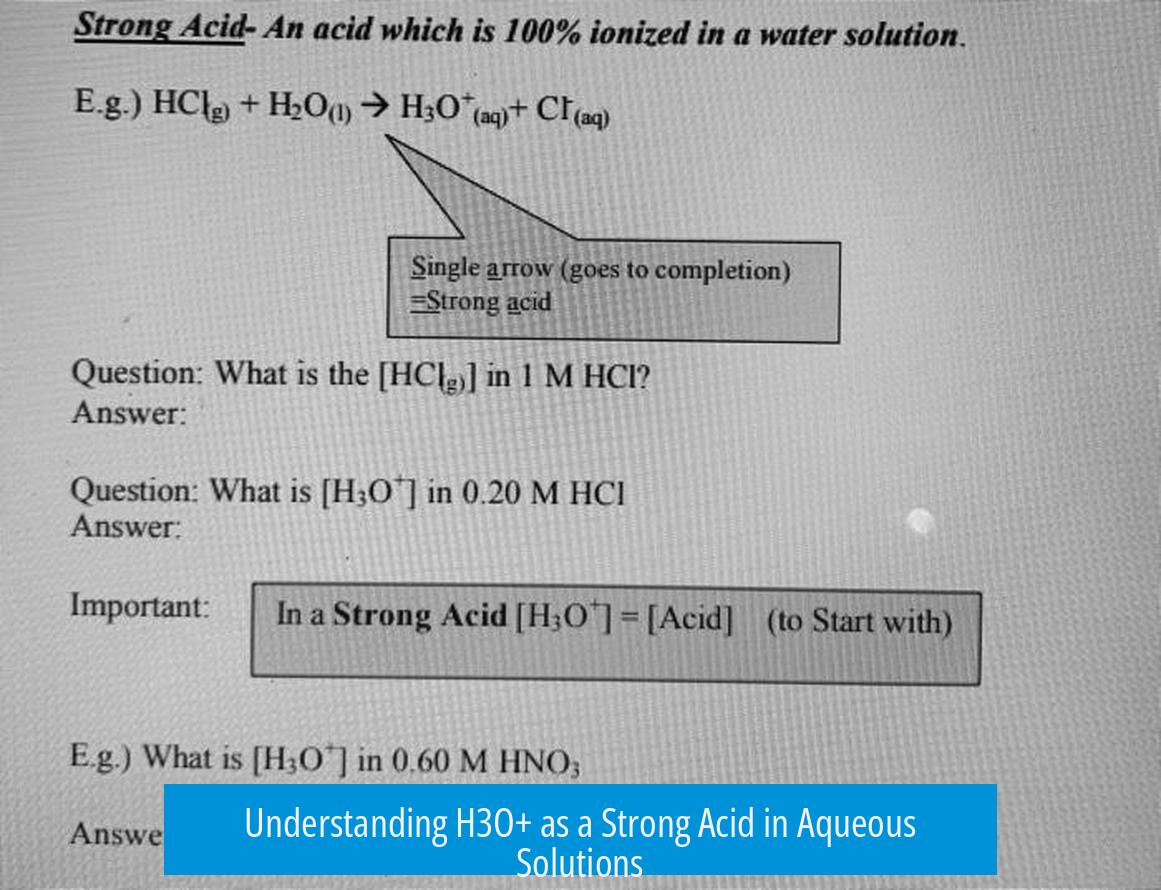
Acid strength depends on how easily an acid donates its proton to the solvent—in this case, water. The stronger an acid, the more completely it donates protons. H3O+ is the conjugate acid of water. Since water is both a weak acid and a very weak base, its conjugate acid counterpart H3O+ inherently has a high tendency to release protons.
Comparing water and its conjugates helps clarify this:
- Water (H2O) has a pKa of about 14, reflecting weak acidity.
- OH– is a strong conjugate base, thus water is a weak acid.
- H3O+, as the conjugate acid of water, is very strong.
When H3O+ donates a proton, it reverts back to water. The acid strength is effectively how readily this process happens, indicating a lower pKa for H3O+ and marking it as a strong acid in aqueous environments.
Equilibrium Constant Formalism
Measuring the acid strength of H3O+ can also be tackled using equilibrium constants (Ka). While unusual since H3O+ itself forms from proton donation, one can define a hypothetical dissociation as:
H3O+ ⇌ H+ + H2O
Since the concentration of water in pure liquid water is about 55 M, and assuming free proton concentration is effectively equal to hydronium concentration, the Ka for H3O+ comes out approximately as 55, corresponding to a pKa of roughly –1.7. This very low pKa numerically classifies H3O+ as a strong acid.
In practice, this formalism helps balance complex acid-base equilibria, charge neutrality, or speciation models in solution chemistry. It does not imply new chemical behavior but offers a quantitative baseline for describing H3O+ acidity.
Acid/Base Equilibria Involving H3O+ in Solution
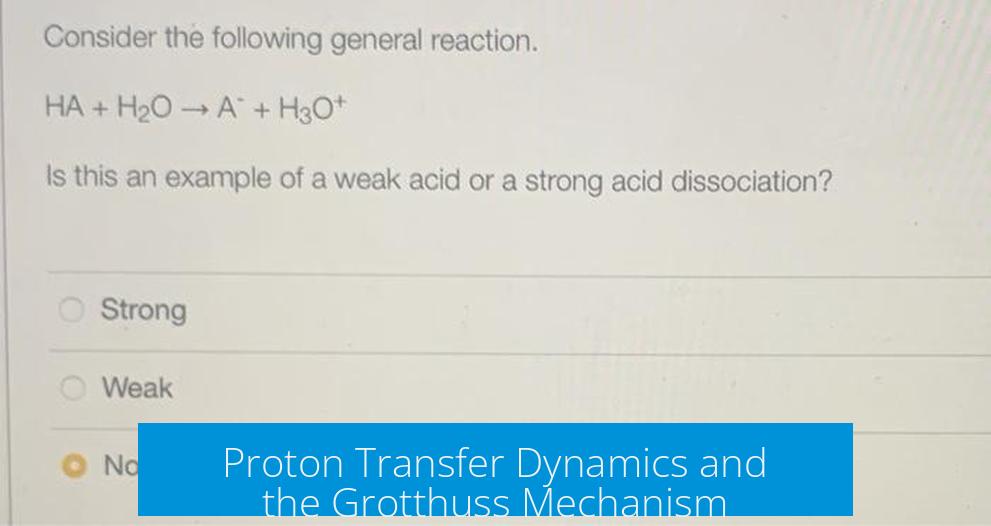
In aqueous solutions, acids donate protons to water, forming H3O+. For example:
HF + H2O ⇌ F– + H3O+
This shows the proton from HF transferring to water, creating H3O+, the actual acidic species in solution. Even acids that dissociate “completely” still produce protons that ultimately reside on water molecules, maintaining hydronium ion concentrations.
Addition of strong acids increases H3O+ concentration, but only to a limit governed by water’s autoionization and equilibrium. Beyond certain concentrations, excess protons simply associate with water, and the system rapidly equilibrates, defining the solution’s pH.
Why H3O+ Is Considered the Strongest Acid in Water
| Property | Explanation |
|---|---|
| Conjugate Pair | H3O+ is the conjugate acid of H2O, a very weak base, making H3O+ very prone to losing protons. |
| Proton Transfer | Protons transfer quickly between molecules via the Grotthuss mechanism, enhancing acid strength. |
| Equilibrium Position | Equilibria strongly favor proton dissociation from H3O+ to water, as shown by a low pKa value. |
| Short-lived Species | H3O+ ions react immediately with molecules in solution, explaining the strong acidic reactivity. |
Additional Insights on H3O+ Acidity
- The designation “strong acid” comes from general chemistry and describes substances with low pKa values and complete or near-complete dissociation.
- The pKa scale itself is relative; acid strength depends on the solvent and conditions chosen.
- H3O+ is the limiting strong acid in water: no acid can be stronger than hydronium in aqueous solution since any stronger acid will transfer its proton to water ions forming H3O+.
- The utilization of H3O+ in reactions simplifies equations and accurately represents proton activity in solution.
Summary of How H3O+ Functions as a Strong Acid
- Free protons do not exist in water; protons form hydronium ions (H3O+).
- H3O+ donates protons readily due to water’s weak basicity, making it a very strong acid conjugate.
- Protons rapidly transfer between water molecules via the Grotthuss mechanism, enhancing acidity.
- The acidity of H3O+ is reflected in its low pKa (~–1.7), underlining its strength.
- Strong acids in water produce H3O+, the fundamental acidic species responsible for low pH and high reactivity.
- Equations often use H3O+ as a proxy for proton activity to simplify and clarify chemical reactions.
Why is H3O+ considered a strong acid instead of H+?
Protons (H+) cannot exist freely in water. They quickly bind with water molecules, forming H3O+. This ion is what actually donates protons in solution, making H3O+ effectively the strong acid species.
How does the Grotthuss mechanism affect H3O+ acidity?
H+ doesn’t move by simple diffusion. Instead, it “hops” rapidly between water molecules. This fast proton transfer means H3O+ can donate protons very quickly, increasing its acidic strength.
What role does water’s weak base property play in H3O+ acidity?
Water is a weak base. Its conjugate acid, H3O+, therefore is strong. This relationship helps explain why H3O+ easily donates protons, making it a strong acid in aqueous solution.
How is the strength of H3O+ shown through equilibrium constants?
The acid dissociation constant (Ka) of H3O+ is roughly 55, giving a pKa near -1.7. This high Ka indicates H3O+ strongly favors releasing protons, confirming its status as a strong acid.
Can the concentration of H3O+ increase indefinitely in water?
No, adding acid only shifts protons onto water molecules, forming more H3O+. However, H3O+ concentration does not grow unlimited because it equilibrates with water, keeping acidity balanced.



Leave a Comment