Is Oganesson a Noble Gas?
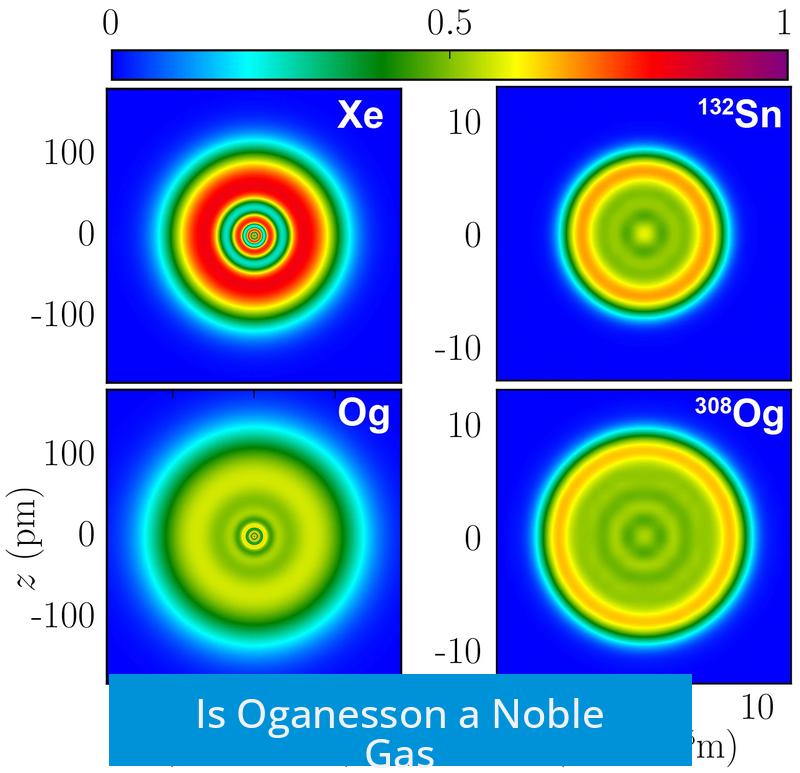
Oganesson is officially classified as a noble gas based on its position in group 18 of the periodic table and its filled electron shell. However, its chemical and physical properties diverge significantly from typical noble gases due to strong relativistic effects, making it less inert and more reactive than expected for this group.
Classification and Periodic Placement
Oganesson (Og), atomic number 118, resides in group 18 of the periodic table, the noble gas group. This group is characterized by having filled outer electron shells, specifically a closed octet, which typically renders the elements chemically inert.
The International Union of Pure and Applied Chemistry (IUPAC) classifies Oganesson as a noble gas primarily on this basis: its electron shell configuration indicates a complete valence shell, a hallmark of the group 18 elements.
Core Noble Gas Characteristics

Noble gases generally exhibit minimal chemical reactivity. Their filled electron shells create stable electronic configurations. This stability reduces the tendency to form covalent or ionic bonds, making these gases largely unreactive.
- Unreactive behavior stems from complete outer shells.
- They have low reactivity with other elements under ordinary conditions.
- The typical physical state is gaseous at room temperature with weak interatomic forces.
Trends in Reactivity Down Group 18
Reactivity among noble gases increases as one moves down the group. Helium and neon are extremely inert, but heavier noble gases such as krypton and xenon display some reactivity under certain conditions.
Xenon, for example, forms several compounds, including xenon hexafluoroplatinate and xenon difluoride. Krypton forms a few unstable compounds as well. This trend originates from the increasing ease of ionizing or oxidizing the outer electrons due to their distance from the nucleus.
Oganesson lies at the bottom of group 18, where this trend predicts even greater reactivity. Its valence electrons are farther from the nucleus and more weakly held, hinting at possible chemical activity rather than inertness.
Relativistic Effects Altering Oganesson’s Properties
Oganesson is one of the heaviest known elements, and its electrons move at speeds close to the speed of light. This invokes relativistic effects that significantly change its electron shell structure.
Relativistic effects result in:
- Increased electron mass, contracting inner orbitals while expanding outer orbitals.
- Weakened shell structure due to altered orbital energies.
- Enhanced spin-orbit coupling that modifies electron behavior.
These changes diverge from lighter noble gases and weaken the typical shell-driven inertness. In Oganesson, relativistic influences strengthen interatomic interactions enough to shift its predicted melting point beyond room temperature, suggesting a solid rather than gaseous state at ambient conditions.
Impact on Chemical Behavior and Physical State
Without accounting for relativistic effects, calculations predict Oganesson to behave similarly to other noble gases, weakly interacting with neighbors and forming no stable compounds.
Including relativistic effects paints a different picture:
- Oganesson atoms exhibit stronger interatomic forces.
- Predicted to have a melting point above room temperature, indicating a solid state.
- Its molecular geometries are unusual, influenced by relativistic orbital alterations.
Thus, Oganesson may not be an unreactive gas but rather a reactive, possibly metallic solid element. Predictions even suggest behavior resembling group 12 metals like mercury or copernicium, defying the noble gas pattern.
Lack of Experimental Data
Oganesson’s extreme instability restricts experimental study. Only a few atoms (approximately five) have been synthesized since its discovery in 2006.
Its half-life measures in milliseconds, preventing chemical characterization. This scarcity hinders direct verification of its predicted physical and chemical properties. Consequently, understanding relies almost entirely on theoretical and computational chemistry models.
Summary of Oganesson’s Noble Gas Status
| Aspect | Oganesson Characteristics | Typical Noble Gas Traits |
|---|---|---|
| Periodic Table Group | Group 18 (Noble Gas) | Group 18 (Noble Gases) |
| Electron Configuration | Filled outer shell | Filled outer shell |
| Reactivity | Predicted to be somewhat reactive | Inert or very low reactivity |
| Physical State | Likely solid (melting point > room temp.) | Gas at room temperature |
| Relativistic Effects | Significant, altering properties | Negligible in lighter noble gases |
| Experimental Data | Very limited, no chemistry studied | Well-studied |
Key Takeaways
- Oganesson is positioned in group 18 and classified as a noble gas by periodic table standards.
- Its filled electron shell supports noble gas classification.
- Relativistic effects significantly modify its electron behavior and chemical properties.
- It may behave more like a reactive post-transition metal than an inert gas.
- Predictions indicate a solid state at room temperature rather than a gas.
- Experimental chemical studies are lacking due to the element’s extreme scarcity and short half-life.




Leave a Comment