Why Do Lone Pairs Form Hybridized Orbitals?

Lone pairs form hybridized orbitals because the atomic orbitals on the central atom mix due to their close energies and the molecular environment, lowering the molecule’s overall energy and increasing stability. This hybridization results from interactions that modify the atomic orbitals, allowing lone pairs to adopt shapes and energies optimal for the molecule’s geometry and bonding.
Understanding Hybridization and Its Models
Hybridization is a theoretical model from valence bond theory designed to explain molecular shapes and bonding patterns.
- Common hybrid types include sp, sp2, sp3, reflecting combinations of atomic s and p orbitals.
- These hybridization schemes simplify complex molecular behavior for easier visualization but do not precisely reflect actual electronic structure.
- For lone pairs, hybridization predicts their location and influence on molecular shape, such as trigonal pyramidal geometry in ammonia.
However, hybridization models are a convenient simplification rather than an exact physical reality.
Molecular Orbital Theory: A More Accurate Picture
The molecular orbital (MO) theory offers a deeper, more accurate interpretation of electron distribution.
- MO theory accounts for molecular symmetry and combines atomic orbitals from all atoms into molecular orbitals.
- Lone pairs occupy molecular orbitals formed by mixing s, p, and sometimes d orbitals, not confined strictly to atomic hybrid orbitals.
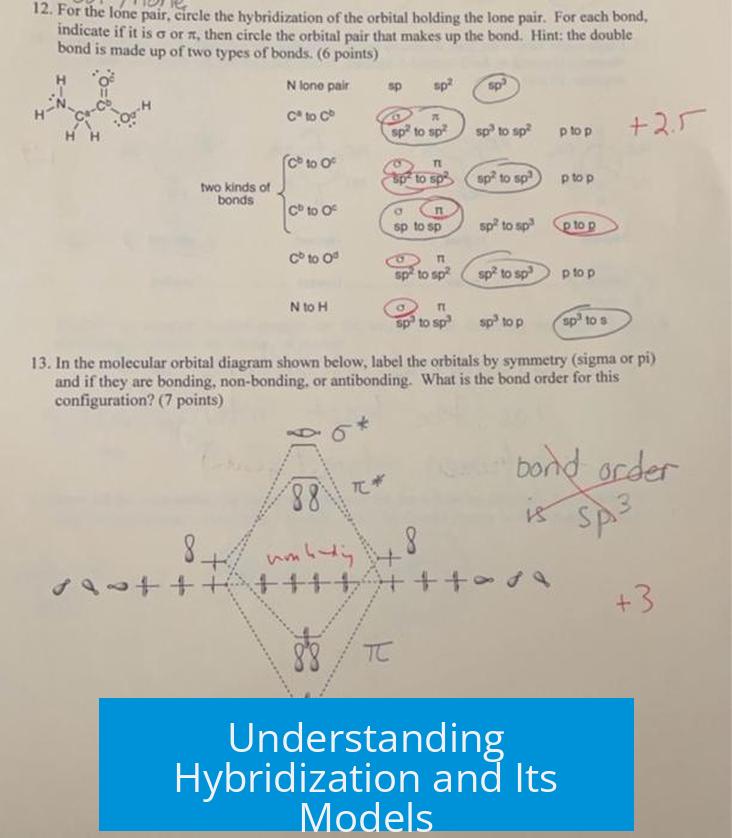
- These orbitals often show mixed character and delocalization, contrasting with fixed hybrid orbitals depicted in valence bond theory.
Lone Pairs and Their Orbital Hybridization
Lone pairs are influenced by the molecular environment, which causes atomic orbitals on the central atom to mix and form hybrid orbitals:
- In ammonia (NH3), nitrogen’s 2s and 2p orbitals have similar energies, enabling significant mixing.
- The lone pair on nitrogen resides in an orbital with mostly p character but mixed with s, often called an sp3 hybrid orbital in valence bond terms.
- This hybridization explains ammonia’s trigonal pyramidal shape and the positioning of the lone pair that affects bond angles.
By contrast, in heavier atoms such as phosphorus, the energy difference between s and p orbitals is larger.
- This reduces mixing, meaning lone pairs retain more s-character.
- As a result, molecules like phosphine (PH3) display bond angles and shapes differing from those in ammonia due to less hybridization of the lone pair.
Energy Considerations Explain Hybridization
The fundamental reason lone pairs form hybridized orbitals is energy minimization:
- The molecular state with hybridized orbitals has lower overall energy than a state where orbitals remain unhybridized.
- Mixing orbitals adjusts electron density to reduce repulsions and optimize bonding arrangements.
- This leads to more stable geometries consistent with experimental molecular structures.
Summary Table: Lone Pair Hybridization Insights
| Aspect | Explanation |
|---|---|
| Hybridization Model | Provides a simple visualization of orbital mixing, aiding prediction of shapes and lone pair position |
| Molecular Orbital Theory | Describes electrons in orbitals involving multiple atoms, revealing true orbital mixing and delocalization |
| Effect of Molecular Environment | Molecular symmetry and bonding interactions cause atomic orbitals to mix, creating hybrid orbitals for lone pairs |
| Example: Ammonia | Lone pair on nitrogen forms from 2s and 2p mixing, resulting in sp3-like hybrid orbital and trigonal pyramidal shape |
| Example: Phosphine | Less orbital mixing due to larger s-p energy gap leads to lone-pair with more s character and less hybridization |
| Energy Minimization | Hybrid orbitals allow the molecule to reach a lower energy, more stable electronic and geometric arrangement |
Key Takeaways
- Lone pairs form hybridized orbitals due to orbital mixing driven by similar orbital energies and molecular environment.
- Hybridization models simplify complex electron behavior and help rationalize molecular geometry and reactivity.
- Molecular orbital theory reveals orbitals are often delocalized and possess mixed character beyond fixed hybrids.
- Energy minimization is the core reason hybridized lone pair orbitals exist, resulting in stable molecular structures.
- Differences in orbital energy gaps among elements (like N vs. P) influence the extent of lone pair hybridization.
I’ve been wondering guys, why do lone pairs form hybridized orbitals?
Wonder no more! Lone pairs form hybridized orbitals because these hybrid orbitals lower the overall molecular energy, making the molecule more stable and vibing at lower energy levels. Now, let’s unpack this with some interesting chemistry storytelling and keen insight.
At first glance, you might recall your old chemistry classes rattling off terms like sp3 hybridization or VSEPR theory with neat diagrams showing lone pairs occupying these “hybrid” orbitals. But here’s the inside scoop: this is mainly a model, a simplified map to help predict shapes and connect dots. Reality, as it tends to be, is more layered.
So why do lone pairs even bother forming these hybridized orbitals? Well, the journey starts with your classic Valence Bond (VB) theory. Hybridization of atomic orbitals helps us explain molecular geometries simply. But it’s important to recognize that these sp3 or sp2 hybrids are a convenient fiction. They do not quite reflect the full quantum dance that electrons perform in molecules.
Hybridization—The Convenient Chemistry Myth
Imagine you’re trying to explain a complex ballet by only describing the dancers’ hats and shoes — it’s helpful but only scratches the surface. Hybridization models, such as sp3, treat orbitals as defined shapes like hats and shoes mixing and matching. They tell you what the molecule looks like, sure, but they fudge the messy reality.
These models come from VSEPR and VB theory that focus on electron pairs repelling each other and orbitals blending for simple shapes. But remember: sp3 hybrid orbitals and VSEPR are more of a classroom tool than mother nature’s exact blueprint.
The Molecular Orbital (MO) Theory: The Real MVP
MO theory takes a deep dive into where electrons actually hang out. It goes beyond the local orbitals around individual atoms to describe electrons spread across the whole molecule — think of it as an electron congregation spanning multiple atoms.
This approach is mathematically complex and demands understanding molecular symmetry to figure out energy levels of molecular orbitals that house bonding electrons and lone pairs alike.
In other words, electrons do not just stick to neat hybrid orbitals on one atom. Instead, orbitals can embody mixtures of s, p, d, and even f atomic orbitals that are delocalized. Usually, they aren’t purely ‘s’ or ‘p’ and aren’t stuck on one atom alone.
This means Lone Pairs (nonbonding pairs of electrons) truly reside in these mixed, spread-out molecular orbitals, which complicates the simpler hybridization picture.
What Happens to Lone Pairs in a Molecule?
Let’s zoom in on ammonia (NH3) for example. You might imagine the lone pair on nitrogen sitting isolated in a p orbital. But no, the nitrogen’s orbitals mix (hybridize) because its 2s and 2p orbitals are close in energy. The result? The lone pair lives in an orbital that’s a mixtape of s and p characters—primarily p-heavy but definitely blended.
In VSEPR and hybridization speak, this translates to an unused sp3 hybrid orbital holding that lone pair. MO theory would explain it as a molecular orbital mostly localized on nitrogen but incorporating the s-p mixing based on the molecule’s symmetry.
The key here is that the atomic orbitals adapt due to their molecular environment. They become hybridized to reduce the overall energy—nature’s way of saying, “let’s chill in the most stable configuration.”
Heavier Atoms Tell a Different Story
Now, what if you swap nitrogen for phosphorus, its heavier cousin? This atom sits lower in the periodic table, and its 3s and 3p orbitals don’t mix as much because their energy difference is larger. The lone pair’s orbital then has more s-character and bonding angles cluster close to 90°.
This illustrates how atomic energy levels influence hybridization. Heavier atoms don’t hybridize their lone pairs as strongly, so lone pairs can look quite different depending on the atom involved.
The Bottom Line: Energy Minimization Drives Hybridization
There’s a simple and profound answer behind every ‘why’ in chemistry: the arrangement you see is the arrangement with the lowest energy. Lone pairs hybridize their orbitals not because nature loves sp3 pyramids per se, but because mixing atomic orbitals into hybrids lowers energy and creates a more stable molecule.
If lone pairs stuck solely to pure p or s orbitals, the molecule would end up higher in energy, less stable, and more reactive—weak stability is like walking on thin ice in molecular terms. Making hybrid orbitals provides a cozy, low-energy seat for the lone pair electrons.
Why Does This Matter? Some Practical Thoughts
- Predicting Molecular Shapes: Hybridization is a quick and useful tool to predict structures like tetrahedral NH3 or bent H2O.
- Reactivity Clues: Lone pairs in hybrid orbitals adopt specific geometries that influence how molecules interact, bind, or react.
- Material Design and Catalysis: Understanding lone pairs’ orbital nature helps chemists design better catalysts or materials based on electronic structures.
In essence, the weird little electrons hiding as lone pairs are not just lazily parked—they’re smartly adjusting their orbital makeup to keep the molecule as happy and energetic-efficient as possible.
So, What Should You Take Away?
Lone pairs do form hybridized orbitals, but this is best seen as a result of atomic orbitals adapting in the molecular environment to minimize energy. While teaching models like sp3 hybridization give you great intuition, the full picture is painted vividly by molecular orbital theory, symmetry, and orbital mixing.
Next time you look at ammonia’s pyramid shape or water’s bent geometry, think about how those lone pairs aren’t just sitting still. They’re in a clever blended orbital, working behind the scenes to keep the molecule comfortably low in energy and ready to rock in chemical reactions.
To sum up:
- Lone pairs hybridize because it lowers molecular energy.
- Hybridization is a simplification; molecular orbitals are complex mixes across atoms.
- Atomic and molecular environments shape how lone pairs’ orbitals form and behave.
- Heavier atoms show different orbital mixing and geometry behaviors.
- The ultimate goal: molecular stability through energy minimization.
So next time, instead of wondering “why do lone pairs form hybridized orbitals?”, you’ll know they are simply playing the best (lowest energy) hand they can, blending orbitals like expert DJs and keeping their molecular party stable!



Leave a Comment