Literally Everything about Hydrogen
Hydrogen is the simplest and most abundant element in the universe, unique in its atomic structure, quantum behavior, chemical forms, and physical properties.

Atomic and Quantum Mechanical Properties
Unique Atomic Structure
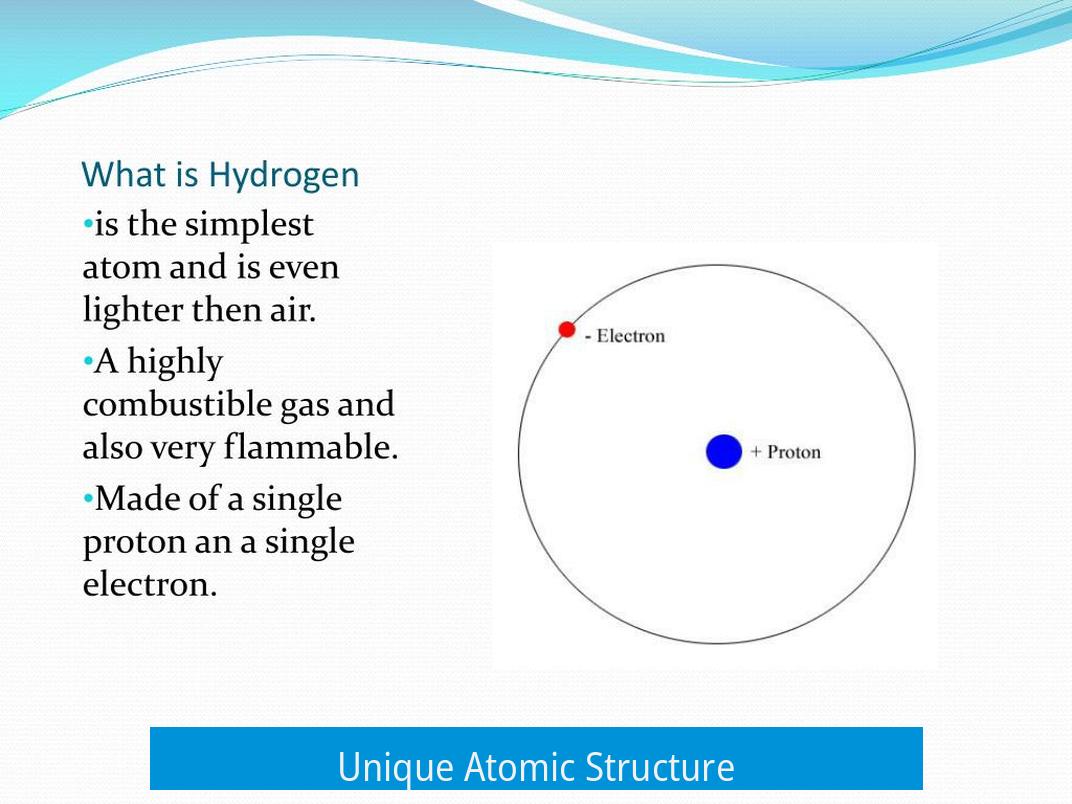
Hydrogen is the only element that forms a stable atom with no neutrons. It consists of a single proton in its nucleus and one electron orbiting around it. This simplicity makes hydrogen the foundational element in chemistry and physics.
Quantum Mechanical Description

The hydrogen atom serves as the primary model in quantum mechanics. It is where the time-independent Schrödinger equation is solved exactly, providing highly accurate wavefunctions and energy levels.
The Schrödinger equation for hydrogen is: Eψ = ((ħ2/2μ) ∇2)ψ – ((q2)/(4πε0r))ψ, where ψ is the wavefunction, ħ is reduced Planck’s constant, μ is the reduced mass of electron-proton system, q is electron charge, ε0 is vacuum permittivity, and r is electron-nucleus distance.
This exact analytical solution is rare in quantum mechanics. The wavefunctions derived from hydrogen’s solution form the basis for understanding electron orbitals in all atoms with more electrons. Other atoms require approximations.
Energy Levels and Experimental Evidence

- The hydrogen energy spectrum revealed subtle quantum effects like the Lamb shift, which confirmed vacuum energy fluctuations.
- Anti-hydrogen experiments showed electronic energy levels match those of normal hydrogen, validating symmetries in fundamental physics.
Spin and Isomers
Hydrogen molecules (H2) exist in ortho and para spin isomers, determined by nuclear spin alignment. Ortho-hydrogen has parallel proton spins, para-hydrogen antiparallel.
- At room temperature, hydrogen is 75% ortho and 25% para.
- Para-hydrogen has slightly lower energy, important in liquid hydrogen cooling processes as conversion releases heat.
- The spin-flip transition between these states emits radiation at 21.106 cm wavelength and 0.704 ns period, a universal reference used in space communication.
Quantum Behavior
Hydrogen’s tiny size means its electron behaves according to quantum mechanics rather than classical physics. It exists as a wavefunction rather than a fixed point and can tunnel through barriers, defying classical logic.
Chemical Forms and Compounds
Molecular Hydrogen (H2)
Hydrogen gas is made of diatomic molecules existing as ortho and para isomers. Unusually, H2 has a negative Joule-Thomson coefficient, warming as it expands, unlike most gases.
Hydrogen gas is highly flammable and explosive in the presence of oxygen. The infamous Hindenburg disaster was triggered by static electricity igniting hydrogen.
Hydride Ion (H−)
Hydrogen can gain an extra electron to form hydride ions, which have a negative charge. These ions often bond with metals, forming metal hydrides like sodium hydride or calcium hydride.
- Metal hydrides act as reducing agents in synthesis and release hydrogen gas when reacting with water.
- Calcium hydride, once used for inflating weather balloons, reacts with water to generate hydrogen safely.
- The hydride ion is comparable in size to the oxide ion (O2-).
Exotic Ions: H3+ and Triatomic Hydrogen
The ion H3+ is a simple molecular ion abundant in interstellar space. It plays a key role in astrochemistry, catalyzing ion-molecule reactions in cold molecular clouds.
Triatomic hydrogen (H3) molecules occur transiently in stellar nurseries where conditions sustain rare hydrogen species.
Physical Properties and States
Metallic Hydrogen
Under extreme pressure, hydrogen transforms into a metallic phase, where protons form a lattice with delocalized electrons. This state exists naturally in gas giant cores and has been recreated in labs using diamond anvil cells.
- Metallic hydrogen might conduct electricity and potentially become a room temperature superconductor.
- In solid form at standard conditions, hydrogen passive diffusion causes metal embrittlement. Metals like palladium absorb hydrogen, losing structural toughness.
Plasma and Gaseous States
Hydrogen ionized in plasma glows purple. The gas itself, when burned, produces nearly invisible flames due to very faint emission light.
Hydrogen is the universe’s most abundant element but occurs mainly as atomic hydrogen or plasma in space, not freely as H2 on Earth.
Production, Reactivity, and Uses
Production Methods
Industrial hydrogen production primarily involves hydrocarbons or carbon displacement reactions. In laboratories, dissolving metals in strong acids generates hydrogen gas effectively.
Catalysis and Reactivity
- Certain metal catalysts, especially transition metals, activate hydrogen in organic synthesis reactions.
- Hydrogen’s high reactivity and diffusivity make it challenging to handle without leaks. Safety remains paramount.
Energy Applications
Liquid hydrogen is a preferred rocket fuel due to its high specific impulse and performance. It boasts the highest energy output in fusion reactions powering stars.
Muon-catalyzed fusion experiments demonstrate hydrogen fusion at room temperature when electrons are replaced by muons, though energy gain does not yet surpass input.
Historical and Linguistic Notes
Etymology
The name “hydrogen” derives from Greek roots “hydro” (water) and “genos” (generator), reflecting early knowledge that burning hydrogen forms water.
Oxygen’s name means acid generator (“oxys” + “genos”), reflecting early misinterpretations linking oxygen to acids.
Historical Uses
Calcium hydride (hydrolith) was extensively used to generate hydrogen for balloon inflation.
The Hindenburg tragedy highlighted hydrogen’s flammability and risk in practical applications.
Isotopes and Related Species
Deuterium and Tritium
- Deuterium (^2H) features a neutron and is found in heavy water, which tastes slightly sweet.
- Tritium (^3H) is radioactive and used in tracer studies and luminous paints.
Exotic Hydrogenic Isotopes
Special isotopes such as muonium and muonic helium arise by replacing electrons with heavier muons. They simulate exotic hydrogen-like atoms with distinct chemical behavior.
Applications in Science and Communication
Nuclear Magnetic Resonance (NMR)
Hydrogen nuclei provide strong NMR signals due to their spin properties, making ^1H NMR the most common technique for analyzing molecular structure.
Space Communication and Universal Standards
Space probes featured the hydrogen atom’s spin state transitions on plaques to define universal units of time and length for potential extraterrestrial intelligences.
- Spin-flip transition: 0.704 nanoseconds period.
- Corresponding radio wavelength: 21.106 cm.
Periodic Table Significance
Hydrogen is element number one, the building block from which all elements can be theoretically synthesized.
Other Miscellaneous Facts
- Hydrogen’s ability to donate protons (H+) renders it a cornerstone in acid-base chemistry (Brønsted acids and bases).
- It was among the first elements formed after the Big Bang.
- Hydrogen is colorless in normal state, but its plasma glows purple due to electronic transitions.
- Hydrogen can diffuse into metals causing embrittlement by altering the metal’s structure and toughness.
Key Takeaways
- Hydrogen is the simplest atom, with unique quantum mechanical solutions and spin isomers.
- It exists in various chemical forms: molecular gas (H2), hydride ion (H−), and exotic ions like H3+.
- Physical states include gas, plasma, and metallic phase under extreme pressure.
- Production methods include hydrocarbon reforming and metal-acid reactions; usage spans from fuel to chemical synthesis.
- Hydrogen isotopes deuterium and tritium have important scientific and industrial roles.
- Spin transitions in hydrogen serve as universal measurement standards in space communications.
- Its high diffusivity and reactivity require careful handling in laboratory and industrial settings.
Literally Everything about Hydrogen: Unpacking the Universe’s Most Curious Element
What makes hydrogen literally everything? Put simply, hydrogen is the simplest, most abundant element in the universe—and a fundamental player in physics, chemistry, technology, and even cosmic communication. It’s a big claim, but buckle up: this post dives deep into hydrogen’s atomic quirks, quantum mysteries, chemical antics, and cosmic roles—all while keeping it lively and digestible.
Hydrogen’s Atomic and Quantum Mechanical Woes (and Wonders)
Hydrogen stands alone in the elemental crowd: it is the only stable atom without neutrons. That’s right—its nucleus is just one lone proton.
Now, the quantum side of hydrogen is a tale told in math and wavefunctions, but let’s keep it neat. Thanks to its simplicity, hydrogen is the rare star of quantum mechanics where the Schrödinger equation actually has an exact, analytical solution. This means scientists can precisely describe the electron’s behavior around a proton, using real math instead of rough approximations—a luxury not often granted for more complicated atoms.
Schrödinger’s Equation for hydrogen looks like this: Eψ = (((h-bar)^ 2)/2μ)(▽^ 2)ψ – ((q^ 2)/(4πε0r))*ψ Where ψ is the wavefunction describing the electron’s state, and all those symbols bring us closer to understanding where that electron might be. It’s a full-on quantum masterpiece.
This exactness isn’t just academic fluff. All those colorful orbital diagrams you’ve seen? They’re derived precisely from hydrogen’s quantum dance, then approximated for atoms with many electrons.
Did you know hydrogen’s energy levels revealed vacuum energy fluctuations experimentally? The Lamb shift is key here, showing how quantum fields gently nudge atomic energies. Plus, antimatter enthusiasts rejoice: anti-hydrogen’s electronic states perfectly mirror normal hydrogen’s energies.
The Spin on Hydrogen Molecules: Ortho, Para, and Galactic Communication
Hydrogen’s quirks don’t stop at atoms. As a molecule (H2), it splits into two spin isomers based on proton spin alignment: ortho-hydrogen (parallel spins) and para-hydrogen (antiparallel spins). At room temperature, a 3:1 ortho to para mixture rules the roost.
Why does this matter? Because when cooling hydrogen to liquids, ortho-hydrogen slowly morphs into para-hydrogen, releasing heat and making the liquid hydrogen boil off. It’s a kind of tiny thermal rebellion.
Intrigued? Here’s a surprise: when NASA sent the Pioneer plaques and Voyager’s Golden Records into space, they used hydrogen’s spin states—both parallel and antiparallel proton-electron spins—to encode a universal scale for time and length. The transition between these spin states has a definitive period of 0.704 nanoseconds and a 21.106-centimeter wavelength. If ET is out there, they should totally get this.
Quantum Behavior: Wave, Particle, or Magician?
Hydrogen’s minute size means it’s better described by quantum waves than little billiard ball electrons. Its location isn’t fixed but a probability cloud. Sometimes it even pulls off quantum tunneling, sneaking through barriers classical physics blesses as impenetrable.
Chemistry’s Favorite Gas: Hydrogen Compounds and Reactions
Molecular hydrogen (H2) is a special gas. Unlike most gases, it heats up when expanding from high to low pressure—a knockout in thermodynamics called a negative Joule-Thompson coefficient. On a less cheerful note, if oxygen shows up, H2 is eager to combust—a dramatic, explosive entrance famously marked by the Hindenburg disaster triggered by static electricity igniting hydrogen gas.
Hydrogen also moonlights as the hydride ion (H−), possessing an extra electron and a 1- charge. This hydride is about as large as the oxide ion (O2−) and often partners with metals like sodium or calcium. These metal hydrides (example: calcium hydride or “hydrolith”) snazzily release hydrogen gas when reacting with water, which once helped inflate weather balloons.
Beyond Earth, hydrogen loves to form exotic ions like H3+, an incredibly abundant molecular ion vital for interstellar chemistry. Even triatomic hydrogen (H3) shows up in the calm nurseries of stars, where collisions happen so infrequently that unstable structures can survive.
From Gas to Metal: Physical Roles and Wild States of Hydrogen
Under incredibly high pressures, like those in gas giants’ cores, hydrogen undergoes a metamorphosis into metallic hydrogen. Here, protons arrange into a lattice, and electrons roam free, creating a metal out of what’s typically the lightest and most well-behaved gas.
This metallic hydrogen phase isn’t just science fiction—it’s been recreated twice in labs using diamond anvils. The excitement? Metallic hydrogen might be a superconductor at room temperature. Imagine your electronics powered with zero resistance fuel, thanks to hydrogen’s pressure-cooked magic.
But hydrogen isn’t always a metal. At room temperature, molecular hydrogen’s flame barely glows; the flame emits such a faint light it’s practically invisible. And if it becomes a plasma, hydrogen shines a soft purple glow, giving off its cosmic signature.
One curious and pesky property: hydrogen causes embrittlement in metals. It creeps through interstitial sites, sneakily weakening metals like palladium, which essentially becomes a hydrogen sponge. This subtle destruction is a major headache in material science.
Making and Using Hydrogen: Chemistry and Technology
Producing hydrogen on an industrial scale often involves hydrocarbons or carbon as reduction agents. Laboratories prefer a classic approach: dissolving metals in strong acids to free hydrogen gas in small quantities.
Thanks to some savvy metal catalysts, hydrogen reactions are a staple in organic chemistry. Active catalysts “turn on” H2, jumping over energy barriers to enable chemical transformations. Some early air-sensitive experiments were conducted in hydrogen atmospheres, although today chemists often prefer argon or nitrogen due to H2’s explosive nature and reactivity.
Leaks? If you’ve ever worked with hydrogen in the lab, you know its tiny molecules slip through seals like water through a sieve. “Guaranteed leak” is practically a motto when handling gas cylinders of H2.
In the realm of space travel, liquid hydrogen is the gold standard rocket fuel. It yields the highest specific impulse, meaning rockets get the most punch per pound of propellant. And fusion? Hydrogen’s fusion powers the sun—the highest energy source known from fusion reactions.
Theoretically, hydrogen can even fuse at room temperature if you replace electrons with muons (heavy electron mimics) in what’s called muon-catalyzed fusion. Sadly, producing muons consumes more energy than fusion provides, so no pocket sun in your lab just yet.
Hydrogen: The Story behind the Name and History
Ever wonder why hydrogen is called that? The name fuses Greek roots: hydro meaning water and genos meaning generator. Early scientists noticed that burning hydrogen produced water, hence “generator of water.” Meanwhile, oxygen (from Greek ‘oxys’ for acid) was thought to create acids. Modern chemistry knows oxygen isn’t essential for all acids, but hydrogen certainly is.
Hydrogen also has a nostalgic place in history. Calcium hydride (“hydrolith”) was used to fill weather balloons before helium became the star. The catastrophic Hindenburg disaster reminds us that hydrogen’s explosive nature is no joke. From early air-sensitive chemistry experiments to modern industry, hydrogen’s story is rich and storied.
Isotopes and Oddities: Deuterium, Tritium, and Exotic Species
Hydrogen wears different hats as isotopes. Deuterium (heavy hydrogen) spices up water molecules with a sweet taste, giving rise to “heavy water.” Tritium, a radioactive isotope, interestingly does not emit alpha radiation, defying certain expectations in physics.
Then there’s the playful world of exotic hydrogenic species involving muons. Muonium is basically an electron orbiting an antimuon—a quirky chemical curiosity acting like a very light isotope of hydrogen. Muonic helium is a similar oddball, chemically behaving like a slightly heavier hydrogen variant.
Science, Space, and Signals: Hydrogen in Communication and Analysis
Hydrogen’s nucleus, with its single proton, plays a starring role in NMR (Nuclear Magnetic Resonance) spectroscopy. Its characteristic spin makes it responsive to magnetic fields, a handy property for chemists probing molecular structures.
On a cosmic scale, NASA’s messages to potential extraterrestrial listeners included hydrogen’s spin transition as a universal standard. The 21-centimeter wavelength signal not only quantifies distance and time but doubles as a cosmic “language” beacon.
Fun Periodic Fact
Hydrogen proudly sits at the very top of the periodic table. With enough hydrogen atoms, you technically have the building blocks to create every other element on the table, through fusion and stellar processes. No wonder it’s termed the universe’s “foundational element.”
Wrapping Up: Why Hydrogen is, Well, Everything
Hydrogen wears many crowns. It is a pristine quantum system, revealing the depths of atomic physics. It’s an explosive, tricky gas critical in chemistry and industry. It’s a space traveler’s ally and a cosmic communicator. From its simplest atomic form to its metallic phase under crushing pressure, hydrogen narrates the tale of matter’s origins and future.
So next time you boil water, ignite a rocket, or scan a nuclear magnetic resonance spectrum, remember: you’re witnessing hydrogen’s quiet brilliance—a tiny atom with a huge story.
Recommended Readings and Resources to Dive Deeper
- Quantum mechanics of hydrogen atom (Lecture Notes)
- Understanding the Lamb shift
- Trihydrogen cation’s role in space
- Curious case of muonium
- Muon atoms explained
- Book Recommendation: Quantum Mechanics of One- and Two-Electron Atoms by Hans Bethe
What makes hydrogen’s atomic structure unique compared to other elements?
Hydrogen is the only element with a stable atom that has no neutrons. It consists of just one proton and one electron, making it unique in atomic structure.
Why is the hydrogen atom important in quantum mechanics?
Its Schrödinger equation can be solved exactly without approximations. This exact solution provides the basis for understanding electron orbitals and energy levels in more complex atoms.
How do ortho-hydrogen and para-hydrogen differ?
They are spin isomers of the H2 molecule. Ortho-hydrogen has parallel nuclear spins, while para-hydrogen has antiparallel spins. Para-hydrogen has lower energy and forms more at low temperatures.
What role does hydrogen play in space communication signals?
The hyperfine transition between parallel and antiparallel proton-electron spins in hydrogen emits a 21 cm wavelength signal. This is used as a universal reference in interstellar communication attempts.
What is metallic hydrogen and where does it occur?
Metallic hydrogen forms under extremely high pressure when protons form a lattice and electrons delocalize. It naturally occurs in gas giant cores but can be created briefly in lab conditions.
How is the hydride ion (H-) different from molecular hydrogen?
The hydride ion carries a negative charge due to an extra electron, unlike neutral H2. It forms metal hydrides used in chemical synthesis and reacts with water to release hydrogen gas.




Leave a Comment