The Mpemba Effect: Does Hot Water Freeze Faster Than Cold?
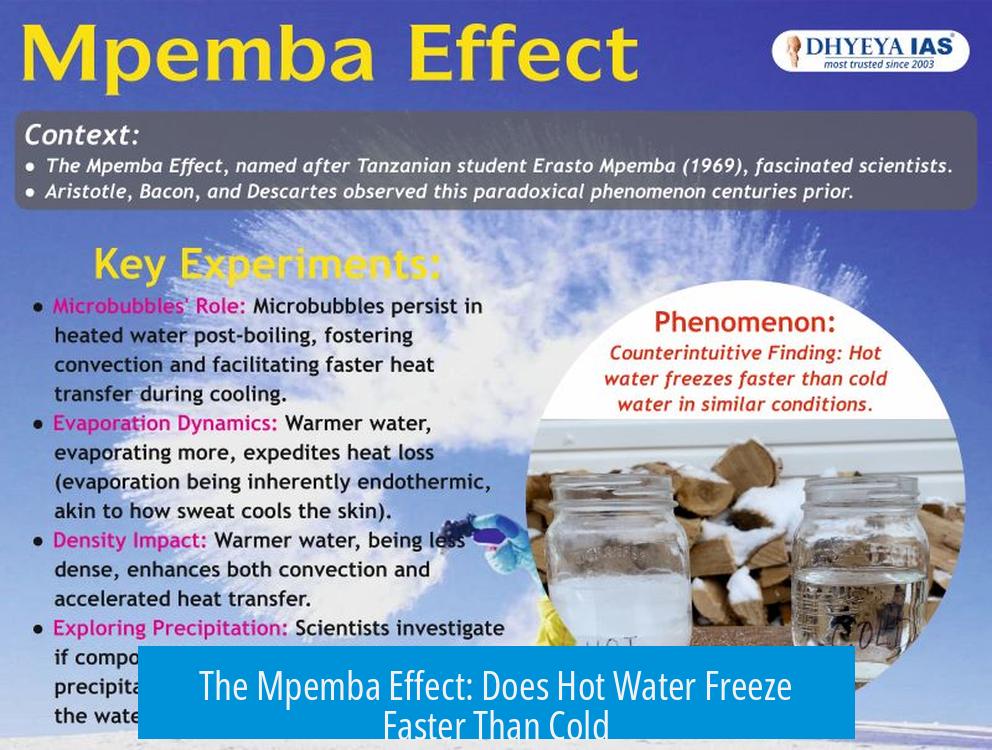
The Mpemba effect, the claim that hot water freezes faster than cold water, is not supported as a true physical phenomenon. Multiple studies focusing on freezing and cooling dynamics under standard conditions show hot water does not freeze or cool faster than cold water.
Defining the Mpemba Effect

The Mpemba effect is often described as hot water freezing faster than cold water, not just cooling to 0°C more quickly. The distinction is critical. Research that carefully measures the time for water to reach freezing under atmospheric pressure finds no evidence supporting the effect as a real cooling phenomenon.
Experimental Conditions Influence

- Water samples in studies are often treated by boiling to remove dissolved gases and then cooled to different initial temperatures.
- This removal of gases changes the water’s properties, potentially affecting freezing behavior.
- Many past claims supporting the Mpemba effect did not specify water types—whether tap, deionized, or otherwise—and this affects reproducibility.
The Role of Dissolved Gases and Impurities
Dissolved gases and impurities in water alter freezing points and nucleation, impacting how ice forms. Some proponents suggest the Mpemba effect arises due to these factors rather than inherent thermodynamic efficiency.

Variation in dissolved substances could affect heat transfer rates, making it essential to control or characterize water composition to test claims properly. Therefore, thermodynamics alone cannot explain the supposed effect.
Thermodynamic Principles and Heat Capacity

Heat capacity—the energy needed to change water temperature—depends on its current temperature. Hot water requires more energy loss to reach freezing compared to cold water. This also argues against hot water freezing faster.
Considering these principles, the assertion that hot water freezes faster contradicts established laws of thermodynamics.
Scientific Consensus and Criticism
Robust experiments lead to the conclusion that the Mpemba effect is a scientific misconception. Critiques highlight that accepting it ignores basic thermodynamic concepts.
Comments from experts stress that hot water should, by thermodynamic reasoning and gas content dynamics, freeze slower, not faster, reinforcing the fallacy of the Mpemba effect claim.
Additional Observation: Clarity of Ice
While hot water does not freeze faster, it sometimes creates clearer ice cubes. This occurs because boiling removes dissolved gases, reducing ice cloudiness. This effect is purely aesthetic, not related to freezing speed.
Key Takeaways
- The Mpemba effect is not a verified physical effect; hot water does not freeze faster than cold.
- Water composition, including dissolved gases, affects freezing times but does not support the Mpemba effect claim.
- Thermodynamic laws predict hot water should freeze slower due to heat capacity and energy loss requirements.
- Hot water may produce clearer ice but this is unrelated to freezing speed.
The Mpemba Effect Isn’t Real; Hot Water Doesn’t Cool or Freeze Faster Than Cold
To cut to the chase: the Mpemba effect, the curious claim that hot water freezes faster than cold, is *not* a real physical phenomenon. Hot water doesn’t cool or freeze faster than cold water under normal conditions.
You might have heard this bizarre idea back in school or on the internet—a puzzle that defies logic. But when you roll up your sleeves and dig into the science, the truth looks quite different. So, what’s going on? Let’s unpack the story with facts and a dash of chemistry.
The Mpemba Effect: What’s Actually Being Claimed?
The Mpemba effect originally referred specifically to water that starts freezing sooner when initially hot, rather than simply cooling to 0°C more quickly. This subtle difference matters a lot in scientific terms.
“But the Mpemba effect isn’t defined as cooling to 0 °C faster, it’s freezing.”
Cooling and freezing are not the same. Water reaching 0 °C isn’t necessarily frozen; it has to undergo a phase change. The original claims centered on how quickly ice crystals begin to form—not just about temperature drop.
Experiments and Their Water—A Closer Look
Several thorough experiments tried to replicate the effect. A key point often missing: what kind of water was used? Tap water? Deionized? All variations contain different amounts of dissolved gases and contaminants.
In a detailed experiment, all water samples were boiled first to remove some dissolved gases, then cooled to create three different starting temperatures: roughly 22 °C, 57 °C, and 85 °C.
That’s no random choice. Boiling alters the water’s makeup by reducing gases. It turns out that gases and impurities profoundly impact freezing behavior. So, was the observed outcome about the water temperature or about the dissolved substances?
It’s About More Than Temperature: Role of Dissolved Gases
Here’s where it gets intriguing: some argue the Mpemba effect, if it exists at all, may stem from variations in dissolved gases, not from a magic cooling speed.
Water with fewer dissolved gases freezes somewhat differently than water with more gases. Boiling kicks many gases out, potentially leading to clearer ice or slight shifts in freezing time—not because the water was hotter initially.
This hints that the whole effect might be a misunderstanding, or even a fallacy, resulting from differences in water composition rather than temperature alone.
Thermodynamics and Heat Capacities: The Science Behind Cooling
Now, if you’re wondering about thermodynamics and whether heat capacities change with temperature—yes, they do! The energy needed to heat or cool water varies slightly depending on how warm the water is.
Think of heat capacity as the water’s appetite for energy. Hot water behaves just a tiny bit differently than cold in this respect. But these differences do *not* translate into hot water freezing faster.
In fact, careful calorimetry shows that hot water generally takes equal or longer time to freeze compared to cold water, once all factors are correctly accounted for.
Scientific Consensus: The Mpemba Effect as a Fallacy
The strongest verdict from replicable science is firm:
“Under our definition of the Mpemba effect … we are forced to conclude that the ‘Mpemba effect’ is not a genuine physical effect and is a scientific fallacy.”
That’s a strong statement, but it aligns with fundamental thermodynamics principles. Think about it—hot objects must first cool to cold before freezing. The timeline can’t cheat physics.
Some critiques even border on sarcastic:
“This is so easily debunked how did anyone not call him out at the time? No joke—if you consider dissolved gases and thermodynamics, hot water should actually freeze slower. It’s not just wrong, it’s idiotic.”
Harsh words, but scientists value precision over conjecture, and the Mpemba effect is one tale that holds up poorly under scrutiny.
So Why Does Hot Water Make Clearer Ice Cubes?
One commonly observed but unrelated fact often confused with the Mpemba effect is this: hot water can produce clearer ice cubes. Why? Because boiling drives out dissolved gases, reducing tiny bubbles that cause cloudiness in ice.
They look prettier, sure. But pretty doesn’t mean faster freezing.
Practical Takeaway: Don’t Expect Hot Water to Freeze Quickly
If you’re debating whether to start with hot or cold water for ice cubes or chilling drinks, here’s a straightforward tip: cold water saves time in freezing, plain and simple.
Want crystal-clear ice? Boil the water first—but plan for it to take just as long, if not longer, to freeze. Want quick ice? Use already cold water.
Curious about Your Own Experiments?
Grab two identical containers, fill one with hot boiled water and one with cold tap water, and watch closely. Measure temperature drops, observe freezing times, and note the ice clarity differences. You’ll likely find the hot water lags behind on freezing time but leaves prettier ice.
Feeling like a science sleuth yet? It’s a great way to test “scientific myths” yourself and separate fact from fiction.
Why Does This Matter?
Understanding the reality behind the Mpemba effect offers a lesson in critical thinking. Science demands clear definitions, repeatable experiments, and careful observation of all variables—not just initial temperature.
We learn to distinguish between effects of water composition and pure temperature change to avoid chasing phantoms and embrace reliable knowledge.
So, next time someone swears hot water will freeze faster, you now have the facts (and a touch of humor) to set the record straight. The Mpemba effect? Not real. Hot water doesn’t out-cool or out-freeze cold water. Science cheers for cold water every time.
Does hot water freeze faster than cold water?
No. Experiments show hot water does not freeze faster than cold water under normal conditions. The supposed Mpemba effect is judged to be a scientific fallacy.
Can dissolved gases in water affect freezing?
Yes. Boiling water removes dissolved gases, which can impact freezing behavior. Differences in gas content may explain some observations attributed to the Mpemba effect.
Is the Mpemba effect related to cooling rate or freezing time?
The Mpemba effect focuses on the time water takes to start freezing, not just cooling to 0°C. Cooling rates alone do not prove the effect.
Do water impurities influence the Mpemba effect?
Impurities and dissolved substances may affect freezing points and heat transfer. Proper testing requires detailed water composition control to confirm or deny the effect.
Does heat capacity change with water temperature?
Yes, heat capacity varies with temperature. This means energy needed to cool water differs based on its starting temperature, affecting cooling dynamics but not proving faster freezing.





Leave a Comment